40 Nutmeg Lane
Glastonbury, CT 06033
Tel: (860) 657-9014
Email: thought@Thoughtventions.com
40 Nutmeg Lane
Glastonbury, CT 06033
Tel: (860) 657-9014
Email: thought@Thoughtventions.com
This is an ongoing, labor intensive work, please be patient for links and updates. E-mail thought@Thoughtventions.com with questions.
In the case of atom additives, attention is usually focused on the light atom group Li, B, Be, and C because these isolated atoms provide a very high heat release upon combustion, and light atoms are energetically favored for propulsion. Whereas light atom implantation of solid H2 has been attempted for a long time, the needed concentrations (5-20%) have not been reached, even in the laboratory.
Atomic Implantation Studies.
Extensive studies have been performed implanting atoms in cryosolid matrices, attempting to create high concentrations of atoms in H2. Boron as a theoretically attractive example of a high energy material that has been cryostabilized at low concentrations. Various processes take place when high-energy boron atoms are introduced into a H2 lattice. Since the boiling temperature of boron is 3980 K, boron atoms fall on the H2 surface with the energy of the same order of magnitude. This energy is insufficient to break apart a H2 molecule (the binding energy is 51,923K) and to form a boron hydrogen molecule, BH. However, this energy is sufficient to melt a region that includes about a hundred H2 molecules. When this local melting happens, intensive diffusion processes take place, leading to recombination of boron atoms to form B2 molecules with an associated energy release (the dissipation energy of B2 is 31,491K - 62,000 cal./mole) that will further accelerate diffusion processes. The density of a boron beam incident on H2 must be small to prevent this recombination of boron atoms.
Another major concentration-limiting effect is that the difference between molar volumes of boron and H2 is very large, so that a strong deformation in a lattice is produced upon insertion of a boron atom. As a rule such deformations create an added effective attraction between impurity atoms and provides a driving force to accelerate diffusion. Where quantum diffusion dominates, it is possible for deformations to hinder diffusion through the disturbance of the coherence of tunneling motion. An accurate and quantitative answer to this possibility can not be obtained without rather complex theoretical calculations that require knowledge about the energy of interaction of boron with H2 molecules, the molar volume of a boron molecule, and an energy spectrum of atomic and molecular of boron in H2 as well. This data does not exist at present. Stabilization of boron solutions in H2 by the introduction of a third substance, for example, deuterium, seems much more probable.
Atoms in H2 Modeling Studies. The computational metastability of atomic impurities, Li and B, in solid para-H2 has been studied by Voth and co-workers [a] employing path integral molecular dynamics (PIMD) simulation at 4 K and zero external pressure. Starting from a pure solid H2 consisting of 1440 particles, doped systems were prepared by substituting impurity atoms for the same number of H2 molecules. For various concentrations, thermodynamic quantities are calculated and the stability of the systems is monitored.
The free energy barrier to recombination was determined to be close to 80 times the thermal energy at 4 K. The effect of the lithium impurities on the matrix phonons was shown to be a minimal effect (a small red shift). This suggested that the phonons of the pure hydrogen could be used to describe the lattice vibrations of the system even with impurities added. A large-scale simulation investigating the limit on the possible maximum doping [a] concentration of lithium atoms before recombination becomes immediate, indicates that at greater than three-mole percent doping the recombination becomes rapid. In addition, the recombination proceeds by a mechanism of initial creation of lithium dimers, which releases heat as well as creating free volume for the hydrogen to reorganize. Recombination of the rest of the lithium atoms follows soon after this step.
A Li system containing 36(2.5mol %) dopants remained metastable, with convergent thermodynamic quantities, whereas a Li system with 48(3.3 mol %) dopants becomes unstable. For the case of boron, a system containing 216(15 mol %) dopants remain metastable while system with 360(25 mol %) dopants does not. These give direct evidence of the transition from metastability to instability, and a rough estimate of the maximum doping density of the two atomic species. The calculations also showed that the boron-doped system stores more energy than the lithium-doped system.
This work must be taken with a grain of salt, since no experiments have either approached the predicted this limit, nor have schemes been developed that could gradually increase the impurity concentration to test the modeling. The atomic interaction description is extremely complex, and will need extensive experimental testing to verify the modeling. Furthermore, non-ideal crystal effects such as vacancies and lattice imperfections may dominate the real process.
The Complications. The above discussions consider the atom and the matrix as two combined named things combined. However much is occurring on the atomic level. The 1440 particles of Voth is simply a calculation of an accumulation of a cube of 12 molecules of H2 on a side with two impurity particles embedded inside, studying the effects of this agglomeration. Considering that the dynamics of the crystal are strongly influenced by long-range forces, the simulation of Voth may misrepresent the real situation in major ways.
A key factor in the interaction between an atom impurity and the H2 crystal is how the atom fits into the crystal. In general the atom can be an interstitial (fitting between the H2 molecules), a substitutional (replacing a H2 molecule), or an additional (replacing more than one H2 molecule) impurity in the crystal. The interaction of the impurity with the crystal and the description of the combination changes dramatically with each type of impurity.
In general the "small" atoms form substitutional impurities, but size does not have fixed meaning on the atomic scale, since it is defined by the intermolecular potentials that vary (usually in 4 dimensions) with the environment in which the atom is placed. This complexity is what makes boron, with its complicated orbital structure, behave far differently in the model than Lithium. At the same time periodic crystal effects may greatly enhance the stability impurity/matrix mixtures, even to the extent of creating van der Waals compounds.
In spite of modeling indications that boron will be a more stable atom in the H2 lattice, lithium has been chosen by Thoughtventions as an initial CSA because:
Calculations [b] show that a H2Li material would allow creation of a fuel with a specific impulse of approximately 481 compared with an Isp of 403 for the standard H2/O2 propellant system. For launch vehicles, a 1% increase in Isp would allow a 9% increase in payload. There are some disadvantages of the choices made. One is the zero energy barrier of Li for reaction, which does not allow true advantage to be taken for concentration by sublimation.
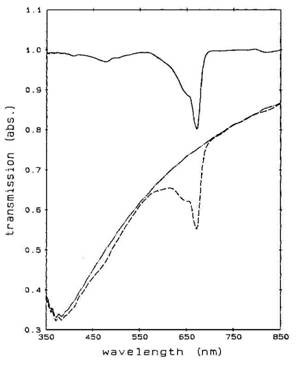
Li Matrix Isolation Studies. The trapping of alkali atoms in cryogenic rare gas matrices has been studied for many years (e.g. [c]). Fajardo [d] has prepared normal-H2 doped with Li impurities by co-condensing a slow flow (0.5 to 1.5 mmol/h) of H2 gas together with the products of a laser ablated lithium plume onto a thin sapphire window cooled to between 3 and 5 K. The inclusion of individual Li atoms in the matrix was demonstrated by studying the optical absorption of the Li atoms. This work provides detailed spectra of the Li/H2 that can be used in the proposed apparatus to determine the concentration of the Li and the progress of the sublimation process. Figure 1 shows the transmission spectra of a Li/H2 matrix and the scattering background due to the H2 matrix itself. The dashed trace is the spectrum of the Li/H2 matrix deposited at T = 3.4 K. The dotted-dashed curve is the transmission of a pure H2 matrix deposited under very similar conditions. It is interesting to note that the deposition occurred above the solid/liquid H2 surface transition temperature. The thickness of these films is not given. The samples were annealed at temperatures below 5.5 K. The details of the causes of the absorption shape are discussed in the publication, and are explained by a combination of different types of trapping sites. This data will allow establishment of a proper sample of Li atoms trapped in solid H2. An increase in the absorption during sublimation will provide a demonstration of concentration. The wavelengths of the spectroscopy are available from standard, inexpensive instruments.
Lithium Source. Designing and building a Li atom source in the context of the thermal source discussed above is not difficult. Lithium [e] is a corrosive alkali metal, but it is the least reactive of all of the alkalis. The temperature at which it changes state is the highest of the alkali metals, (melts @ 180°C, boils @ 1326°C) but these temperatures are easily achieved by a resistive thermal source. Some characteristics of Li will require handling with care; especially its reactivity with N2.
Please e-mail Steve Bates at thought@Thoughtventions.com to discuss this research.
Last updated: July 2015