SHEAR STRENGTH TESTING OF SOLID OXYGEN [a]
S. C. Bates* and T. L. Altshuler**
* Thoughtventions Unlimited, 40 Nutmeg Lane, Glastonbury, CT 06033
**Advanced Materials Laboratory, Inc., 242 Baker Ave., Concord, MA 01742
Experiments have been performed to test the shear strength of solid oxygen. Shear strength measurements were made in a cryostat by pulling a rod out of solidified liquid oxygen. The approximate shear strength of solid oxygen was measured as a function of temperature, increasing from 0.31 MPa at 45 K, to 4.46 MPa at 18 K. Solid oxygen was found to undergo plastic deformation at high temperatures, becoming increasingly strong and brittle as its temperature is decreased. Data and simple experiments confirmed a similarity of engineering material properties between solid oxygen and room temperature plastics.
INTRODUCTION
Solid oxygen is a member of a fairly small set of related cryogenic solids that are most accurately described by the weak Van der Waals binding forces between their molecules. Van der Waals solids include molecular hydrogen, oxygen, nitrogen, the "noble" elements and some others. Solid oxygen is a paramagnetic bluish crystal that forms when liquid oxygen is cooled below 54 K. The solid is not a simple crystal, but exists in 4 different forms (1). Most of the research done on solid oxygen has been done as a result of the theoretical interest in the unique magnetic properties that result in its different structural forms. Its phase transition temperatures have been considered as secondary fixed points in thermometry. The major crystal phases solid oxygen (1) are the cubic γ-phase existing from 54.4 K to 43.8 K (sp. gr. = 1.39), the rhombohedral β-phase from 43.8 K to 23.9 K (sp. gr. = 1.50), and the monoclinic α-phase below 23.9 K (sp. gr. = 1.55). The detailed macroscopic properties of solid oxygen are difficult to predict theoretically (2).
Although the basic structural and thermodynamic data for solid oxygen has been known for quite some time, the engineering properties of the solid are almost entirely unknown because of limited interest and the difficulty of cryogenic testing. The microscopic structural and thermodynamic properties of solid oxygen have been both calculated theoretically (3) and derived from macroscopic experiments. Some of the engineering properties of the solid could be derived from fundamental known properties in principal, but the results would be modified by polycrystalline behavior. An example of this difficulty is that the α-phase of solid oxygen has yet to be made as a single crystal. Furthermore, most properties will be anisotropic as a result of the crystal structure. Knowledge of the engineering properties of solid oxygen is crucial for creating innovative practical solutions to the problems of using a solid cryogen in both large and small quantities. Although liquid cryogenics is a mature field, solid cryogenics is almost totally unexplored.
The closest approach to an application for solid oxygen has been to use its higher density and longer storage time for the supply of oxygen for life support. In this case it has significant advantages over the liquid and supercritical fluid for long term storage (4). The added heat of fusion, heats of transition, and additional sensible heat of the solid leads to much longer storage times for the same heat leak. The relevant parameters are that the heat of fusion from the liquid to the gamma phase solid is 106 cal/mol, and the heat of transition from the gamma to the beta phase is even larger: 178 cal/mol. The heat of fusion to the alpha phase is less, so that most of gain in heat of fusion and density is achieved at 25 K. The solid also has a significantly lower volume than the liquid. Cooling liquid oxygen to a solid just below the triple point gives a 13.9% increase in density, improving to 22.4% at 30 K, and 28.1% at 20 K. The storage application was eventually rejected because no practical means could be found to transfer the solid.
Relevant and important past cryogenic engineering work with solid oxygen includes work at Aerojet-General on cryogenic oxygen storage (4), production of slush oxygen and hydrogen at NBS (now NIST) (5), and studies of the magnetic properties of solid oxygen (6). There is also currently ongoing research (including that reported here (7)) in solid oxygen and hydrogen cryogenics being done at the Air Force Phillips Lab and, through their funding, at various universities. This work is motivated by the desire to stabilize more energetic oxidizers in a solid oxygen matrix to obtain a higher energy density propellant. Although the solid mixture holds the promise of greater energy release, the use of the cryogenic solids themselves creates new engineering problems.
SIMPLE SOLID OXYGEN EXPERIMENTS
The need for fundamental data on the mechanical properties of solid oxygen arises from design questions about the feed and injection of the solid for a propulsion system. The shear strength must be known to design an extrusion device, and since oxygen is known to be abrasive, some information is needed about how this abrasiveness will affect the design. Some simple experiments were thus attempted to get a preliminary estimate of the shear strength of solid oxygen. Estimates of shear strength based on the literature and experience implied a value as high as 7 MPa, but based on hydrogen pellet extrusion experiments that measure a 0.5 Mpa, shear stress for high temperature solid hydrogen, the actual strength of solid oxygen was expected to be closer to this latter value.
Common experience with a Van der Waals solid is primarily with the inconvenience these solids cause when they occur accidentally in a liquid cryogen system. Examples are solid air in the bottom of a helium, dewar or solid nitrogen blocking a nitrogen gas line. Pressures of 1 to 2 MPa are not enough to clear a blocked line, suggesting the possibility that estimates of shear strength could be made by measuring the pressure required to clear a line of a solid blockage. A thin diaphragm would be used to contain a known volume of liquid nitrogen/oxygen/air in a tube. This liquid would then be frozen by lowering the tube into a liquid helium dewar. The helium pressure required to dislodge this solid from the smooth tube would give a measurement of the shear strength. Designing such a simple experiment was in fact very difficult. The primary problems were the possibility of an explosive discharge when solid oxygen breaks, and the temperature sensitivity of the measurements. The heat transfer to the walls of the tube is sufficient to quickly melt the solid oxygen adhering to the wall and negate any test results. This implies the need for shielding the end with helium and liquid nitrogen, which in turn leads to complications for an explosive pellet discharge.
The analysis of these problems suggested alternate experiments using nitrogen instead of oxygen. Nitrogen does not have the fire hazards of oxygen and has similar freezing temperatures and molecular forces, so that its strength and behavior should be similar to that of oxygen. By crimping the end of a stainless steel tube and pushing a rubber stopper down to the end, a freezing cup was created. Liquid nitrogen (LN2) was then poured into the tube and allowed to solidify at the bottom (on the order of an hour). A solid nitrogen test consisted of 1) quickly withdrawing the tube from the dewar, 2) inverting it over a LN2 cooled block, 3) Waiting a few seconds until the heat transfer to the tube melted the side of the solid nitrogen plug and it dropped out of the tube, 4) quickly manipulating the plug to a test position, and 5) performing a short mechanical or visual test on the plug. The plug survived about 5 seconds before total evaporation. Clear, condensation-free, visual access was obtained by taking advantage of the density stability of the cold gas that prevented water vapor from interfering with inspection through the open top of the apparatus.
The most interesting insight gained by manipulating these plugs is that they are clearly plastic at higher temperatures. In the case of a cylinder placed between two supports over a LN2 bath, it would initially maintain its position in response to a downward force at the center and then bend into a U shape. A similar plastic behavior was seen in response to impact with a hammer. The cylinder would squash but never shatter or splatter. This was very surprising behavior that implied that the solid (nitrogen) is a plastic material of moderate strength. These observations confirmed the behavior of the solid during the more sophisticated shear tests. The solid can be clear or white, depending on the condensation history - the white color arises from included voids.
CRYOSTAT SOLID OXYGEN SHEAR EXPERIMENTS - APPARATUS
A cryogenic mechanical testing apparatus (8) was used to measure the shear forces needed to move a metal rod embedded in solid oxygen. A Sintech Model 2000-6 computer-integrated mechanical strength testing system was used in combination with a Janis 10CNDT cryostat capable of operation between room temperature and 1.4 K. A Lake Shore Cryotronics 805 temperature controller was used to monitor and control temperatures within the cryostat , using a silicon diode temperature sensor (Lakeshore Cryotronics DT-470-SD-13, accuracy of ± 1 K) clamped onto the bottom of the copper oxygen container (cup). Figure 1 shows a schematic of the assembled apparatus. The cryostat is a standard model with vacuum insulation between a liquid nitrogen dewar surrounding a liquid helium dewar, in turn surrounding a gaseous helium test volume that contains the mechanical testing apparatus.
The primary design difficulties for this experiment were to: 1) develop an apparatus that could be easily assembled yet support significant mechanical shearing forces, 2) fit the apparatus within the small cryostat test volume (5 cm diameter, 12 cm long), 3) provide enough heat capacity and thermal conductivity to liquefy then solidify the oxygen in a reasonable time, 4) distribute the loading on the solid to give accurate shear measurements, 5) provide entry and exit for the oxygen gas through the cryostat's thermal barriers and make the flow rate high enough to fill the cup relatively quickly, 6) provide a precisely metered oxygen supply source, 7) contain the oxygen so it only condenses in the cup and not on the colder surfaces of the dewar, and 8) provide temperature monitoring and temperature control to be able to perform experiments at different temperatures. Procedures also had to be developed to feed the oxygen gas, liquefy it, solidify it, and then achieve the test temperature.
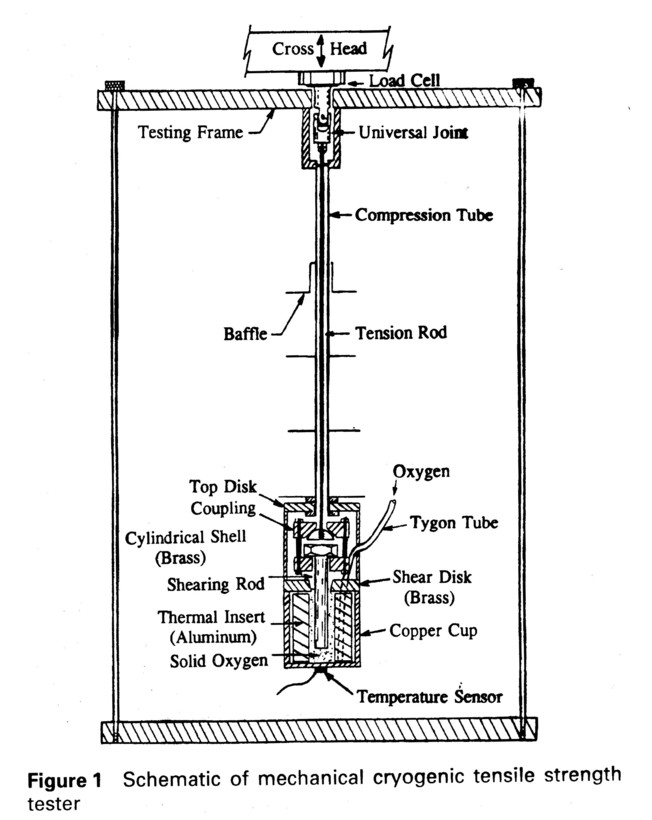
Some of the lessons learned were: 1) The gas feed tube is easily blocked if its temperature is below the oxygen solidification temperature, 2) The oxygen is density stable in a cold helium bath and the gas diffusion rates are very slow, so gaseous oxygen can easily be kept in an open cup without significant losses to condensation on farther away colder surfaces; a vacuum container is not needed, 3) To minimize solidification time it is necessary to have enough heat capacity in the supporting structure to solidify the oxygen than to rely on convective and conductive heat transfer from cold external helium gas, 4) Thermal equilibration times for the solid oxygen can be long if the solid dimensions approach 1 cm, making experiments long, 5) The assembly, thermal, structural, space, liquid containment, shearing geometry, and motion constraints on the shearing apparatus lead to a very complicated design, 6) Proper condensation of the oxygen is crucial. Oxygen condensation on parts of the cryostat where it was not wanted prevents accurate filling of the shearing volume and can lead to an explosion in the vacuum pump during pumpdown. Condensation in the shearing apparatus might also cause additional solid contact that would confuse the shear measurement.
An important aspect of the solidification of oxygen is the supply of thermal energy needed to solidify the oxygen from the gas. Cooling through solid oxygen is very slow, so the volume of oxygen to be used was minimized by a metal insert in the solidification cup to keep the solidification time as short as possible. The insert also absorbed much of the heat of condensation. On a volume basis the specific heat of available materials at 67 K does not vary with material, so the most convenient material - aluminum - was chosen. Care was taken to assure that shearing did not take place against the aluminum. The oxygen needed to fill the cup was estimated to result in a 15-20 K warm up of the structure, such that if solidification is begun with the shearing apparatus at 60 K, the structure remains cold enough to keep the oxygen from boiling (90 K), after which the liquid could be solidified by thermal conduction. The minimum temperature of the structure was 55 K to prevent blockage of the gas inlet tube by solid formation (this happened quickly in practice at lower temperatures).
The overall shearing apparatus (Fig. 1) consists of an external shearing frame with a concentric tube and rod provide the shearing forces; the outer tube is in static compression, whereas the inner rod is moved in tension to steadily increase the shear force applied at the end of the tubes within the cryostat. The configuration of the cryogenic shearing fixture is also shown in Fig. 1. This fixture consists of an inner central tension fixture designed to assure strictly axial loading of the puller rod in the solid, and an outer stress-transmitting shell ending in the oxygen cup. Brass, copper, and aluminum are the materials of choice for use with oxygen. The copper cup was sized to accommodate a wide range of shearing diameters, depending on the actual shear strength of the oxygen. Sealing was minimal, using only as-machined metal to metal contact. The shearing plate was shaped to provide a shearing edge and to assure that overfilling the cavity with oxygen will not affect the shear results. The central rod was a commercial high strength stainless steel 1.25 cm diameter bolt.
A set of experiments were performed in this apparatus using different puller rod materials to determine friction/adhesion differences and using a multiply slotted rod to shear oxygen to obtain an accurate measurement of the inherent shear strength of the solid. Measurements versus temperature were done to explore the temperature dependence of the shear strength.
PROCEDURES
Oxygen for solidification was supplied from a 20.00 liter tank whose pressure was measured by a pressure gauge with an accuracy of 1.5%. This tank was filled from a standard, high purity oxygen cylinder and was warmed to room temperature after gas transfer to eliminate the effects of expansion cooling during gas transfer. The tank was connected to the cryostat with a 0.1/0.3 cm ID/OD tygon tube, which was threaded down the inside of the cryostat through holes in the thermal shields ending at the bottom of the copper cup. Since the tube end was in the bottom of the condensation cup any gas reaching the end liquefied as it bubbled up though the liquid oxygen already in the cup. The liquefaction, solidification, and shearing processes were all performed under one atmosphere of helium. Oxygen was bled into the cryostat for 5 - 10 minutes while the temperature of the copper cup was monitored to be sure it was much colder than 90 K, keeping the oxygen vapor pressure low. In practice the transfer was begun at a structure temperature of approximately 60 K; during condensation this temperature never rose above 70 K. Before assembling the apparatus the volume in the copper cup was measured by filling with water and weighing, as was the volume of the gas tank.
For each puller geometry repeat tests and tests at different temperatures were performed by shearing the solid, warming to liquefy, replacing the puller, solidifying again, and shearing again. After a series of tests the apparatus was allowed to warm up to room temperature after boiling off the cryogenic liquids, and the oxygen was vented to the room. The specimen chamber that contained the oxygen was always first flushed with helium if evacuation was necessary.
After the oxygen testing apparatus was assembled and inserted into the cryostat, the cryostat was first cooled to 115 K with liquid nitrogen. Liquid helium was then transferred into the cryostat. When the temperature of the copper oxygen container reached about 65 K, oxygen gas was bled into this container through the tygon tubing and liquefied. A precise gas volume of oxygen was added so that the volume of the desired liquid oxygen would fill the oxygen container to a liquid level about a millimeter above the bottom surface of the brass shear disk (G in Fig. 1). The condensed oxygen is shown as item (E) in the figure. In the case of the cylindrical bolt and the shear rods, 18 ml. of liquid oxygen was required. When the radially slotted shear rod was used, 20 ml. of liquid oxygen was used. A pressure drop of 78 kPa in the 20 liter storage tank resulted in a transfer of 15.5 liters of oxygen at STP, which condensed to 18 ml. of liquid oxygen.
During liquefaction of oxygen, the temperature was maintained between 62 and 68 K by bleeding appropriate amounts of liquid helium from the cryostat reservoir through a capillary tube into the testing chamber. This temperature was selected to be as far as possible below the vaporization temperature of 90.19 K yet safely above the solidification temperature of 54.36 K (9). The heat required to be removed from oxygen at 300 K and to condense it at atmospheric pressure was 3,110 joules/mole or 174.9 joules/cm3 of liquid based on the Mollier diagram for oxygen (9). Thus, 3,149 joules were required to cool room temperature oxygen to 18 ml. of liquid at 90 K. It was estimated that the rise in temperature of the metal containing the liquid oxygen based on its heat capacity would have been about 15.5 K had there been no bleeding of liquid helium into the specimen chamber.
After liquefying the oxygen, the test chamber was cooled to the desired testing temperature and held at that temperature for 15 minutes to ensure that the solid oxygen was at the same temperature as the copper oxygen container, as measured by the silicon diode sensor. The elapsed time between a phase change and a test was typically on the order of 30 minutes. A mechanical test was then performed. After shear testing the solid oxygen at a strain rate of 0.25 cm/min to failure or to a sufficiently large strain, the temperature of the oxygen container was raised to about 65 K and held there for fifteen minutes to reliquefy the oxygen. Then the oxygen test apparatus was recooled for an additional test. The solubility of gaseous helium in liquid/solid oxygen is small; the presence of helium is not believed to affect the results of testing.
RESULTS
Solid oxygen shear tests were performed using 1) a stainless steel bolt with standard coarse threads, 2) a smooth stainless steel smooth rod, 3) a teflon (TFE) coated stainless steel smooth rod, and 4) a shear rod with multiple grooves.
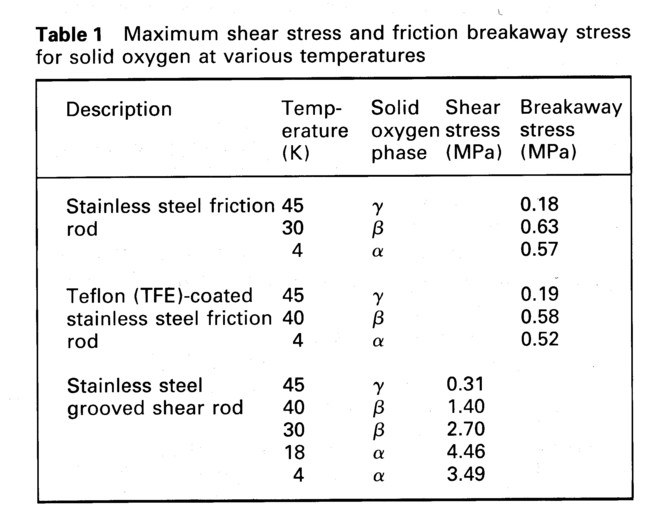
A stainless steel bolt was used for preliminary tests to assess the type of deformation that would occur during shear tests of solid oxygen so that further experiments could be designed more accurately. The bolt had a shaft diameter of 1.252 cm with threads (American National Coarse - 13 threads per inch) extending for a length of 3.175 cm. The bolt was immersed in condensed oxygen, beneath the shearing edge for a length of 1.78 cm beyond the top of the threads. Since the solid γ oxygen phase exists between 43.80 K and 54.36 K (9 two tests were performed at 45 K. The stress versus displacement results of these tests were characteristic of a standard plastic material, with a peak load of 326 N and 512 N. The load in both cases reached the peak yield point, decreased somewhat, and then the bolt slipped continuously until the test was stopped.
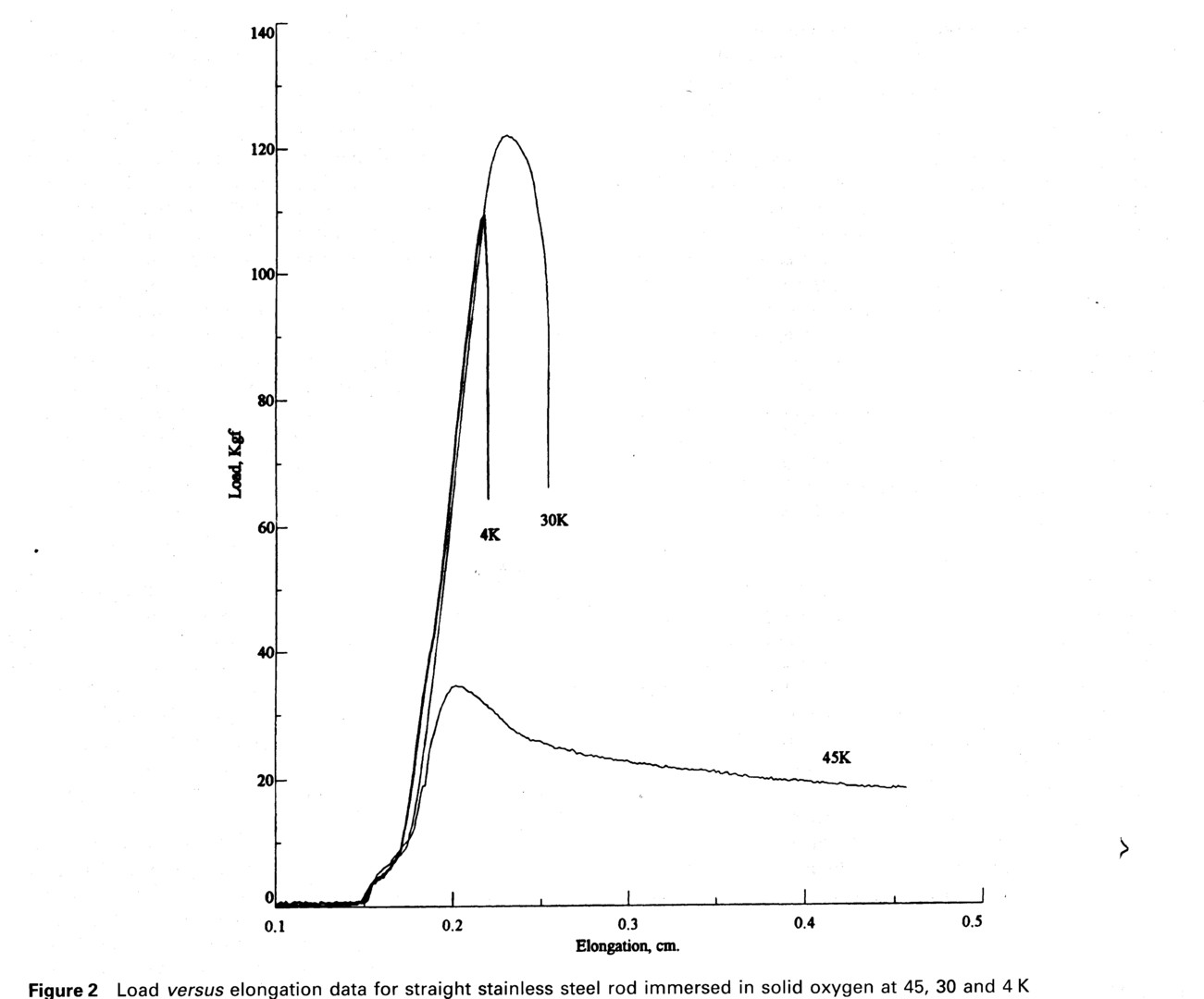
A stainless steel bolt was next cut off above the threads and its surface smoothed with 600 grit silicon carbide paper. The diameter of the rod was 1.247 cm. It was immersed 4.83 cm into the liquid oxygen, giving an oxygen shear area of 19.0 cm2. Data from smooth rod shear tests performed at temperatures of 44.8 K, 30.0 K, and 4.2 K are shown in Fig. 2. At 44.8 K, for the solid γ oxygen phase, the peak load of 339 N was practically the same as the first test using the threaded bolt. Based on the calculated oxygen shear area this is equivalent to a shear stress of 0.18 MPa. Plastic deformation continued until the test was stopped. For the solid β phase oxygen test at 30 K the load reached a peak load of 1197 N and a shear stress of 0.63 MPa, rolled over, and after dropping 40% the bolt broke free from the solid oxygen. At 4.2 K for α phase oxygen, there was very little plastic deformation after the sample yielded. A peak load of 1072 N was reached with shear stress of 0.57 MPa, and then after slightly deforming, the rod slipped free of the solid oxygen. The data shows a continuous progression from fully plastic to fully brittle behavior as the solid temperature decreases from the melting point to 0 K.
A stainless steel bolt, similar to the one used previously as a smooth rod, was coated with a 0.0025 cm thick layer of teflon (TFE). Testing conditions with the coated rod repeated those of the uncoated rod. Three smooth rod tests were performed, one each at 44.8 K, 30.0 K, and 4.2 K with the results shown below in Table 1 giving shear stresses derived from peak load values as before.
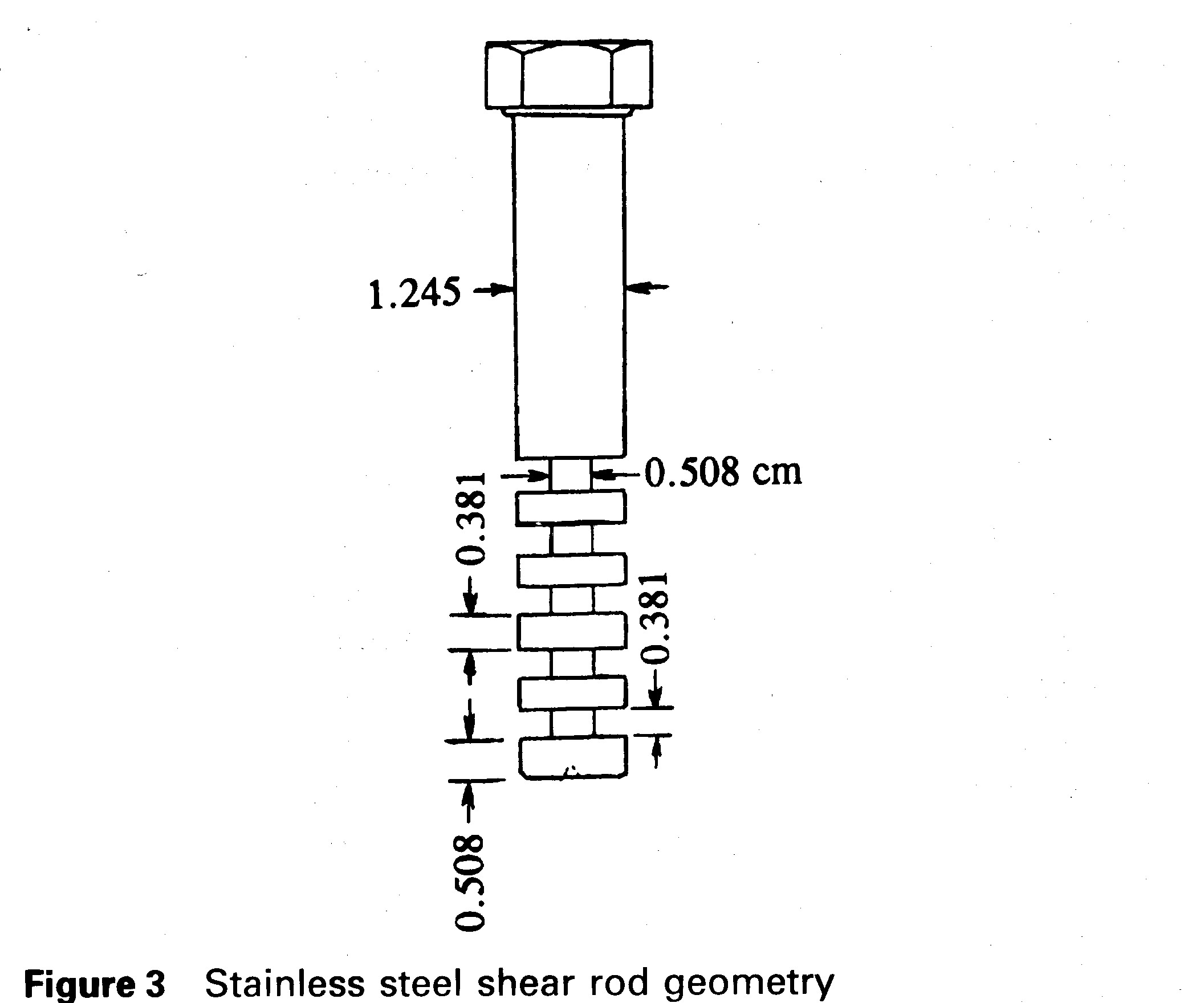
A stainless steel slotted shear rod with the geometry shown in Fig. 3 was used to provide shear testing data for bulk solid oxygen. This design was deemed to be the best available simple geometry that would give an approximation to an accurate strength value. The shear rod was again immersed 4.83 cm in liquid oxygen. The surface area between the outer diameter of the rod and the solid oxygen was calculated to be 11.93 cm2. The shear area where the solid oxygen entered the grooves of the rod was calculated as 7.42 cm2.
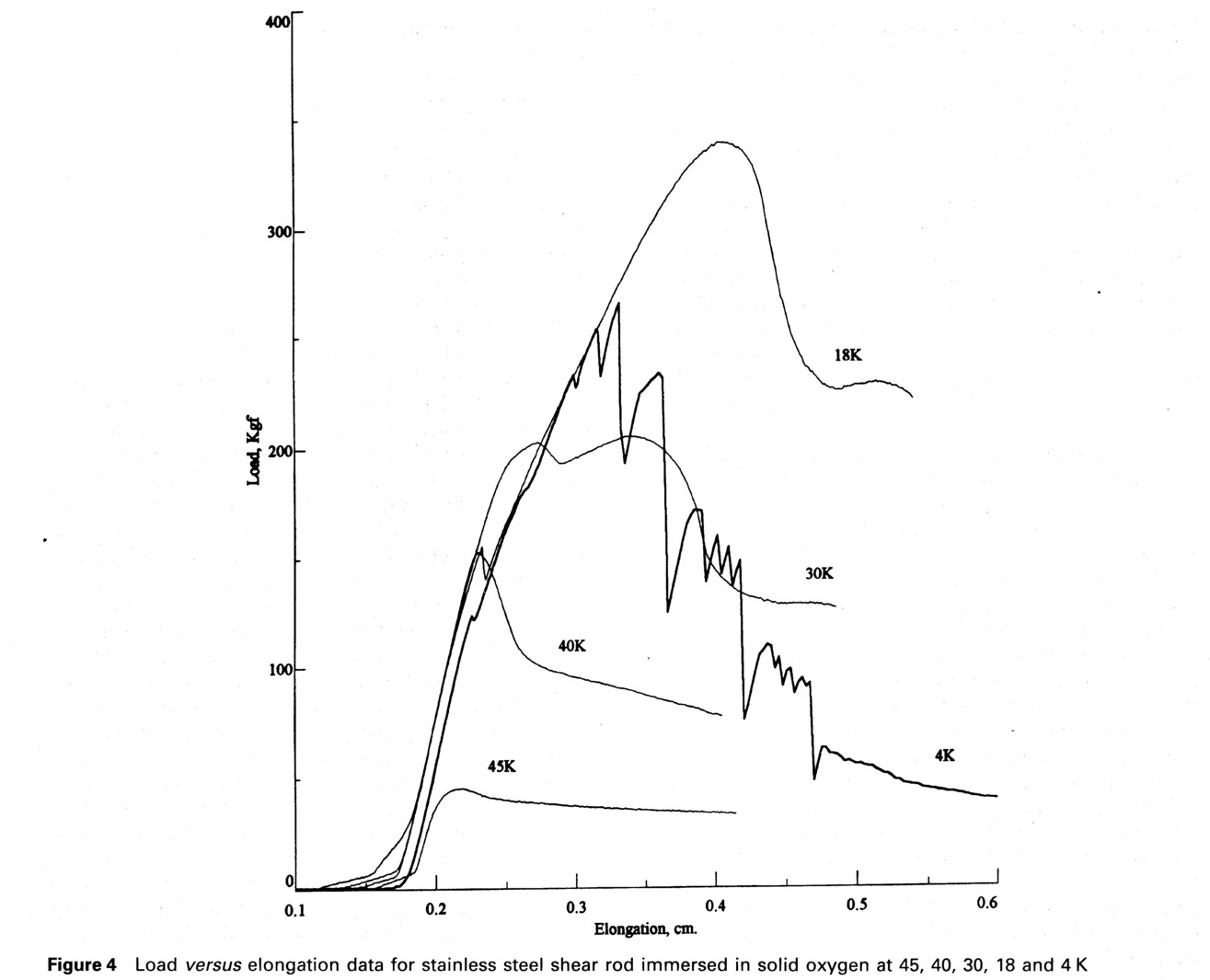
The results of shear testing at a range of solid temperatures are presented in Fig. 4. Shearing at the outer part of the bolt is not believed to contribute to the peak shear value except for the data taken at 45 K, because the shearing elongation had caused the solid oxygen to break free from the bolt surface well before the peak shear stress was reached. The breakaway event is indicated by the first load drop in the curves, which corresponds closely to the elongation measured in the smooth rod tests. This is discussed more fully below. The exception is the γ phase oxygen at 44.8 K where the load showed no drops and peaked at 445 N at the same elongation as the smooth rod tests, afterward decreasing gradually during plastic deformation. For the 45 K data alone the maximum shear stress was thus calculated to be 0.31 MPa, based on the groove area of the bolt, after subtracting a 213 N contribution of the shear between the outer rod diameter and the solid oxygen using the smooth rod test data adhesive strength at this temperature.
At lower temperatures the bulk shear strength is calculated based only on the peak load and total groove area, 7.42 cm2. At 39.7 K for β phase oxygen a peak load of 1500 N was reached giving a maximum shear stress of 1.40 MPa. For the 29.3 K test of β phase oxygen, the first peak load was 1980 N, and the second peak load of 2010 N was observed at a larger elongation, corrsponding to a shear stress of 2.70 MPa. There was considerable further plastic deformation of this solid oxygen with a drop in load at which time the test was stopped and the rod was unloaded. When a test was performed on α phase oxygen at 18.3 K, there was a load drop at 0.23 cm elongation in Fig. 4 at 1530 N load. At the peak load of 3310 N, the shear stress was calculated to be 4.46 MPa. There was then continued plastic deformation of the solid oxygen with a steady drop in load to a point, when the test was stopped. The final test at 4.2 K was shows complex behavior. Multiple drops in load occurred before and after a peak load of 2600 N was reached, which is equivalent to a shear stress of 3.49 MPa.
The shear strength results of all of the tests are presented in Table 1 below. The magnitude of the shear strength of solid oxygen is low (0.31 MPa) at the higher temperatures but increases to moderate values (4.5 MPa) in the alpha phase, increasing by a factor of ten in strength. At higher temperatures there was considerable plastic deformation of solid oxygen, whereas at 4 K, its behavior is primarily brittle. Coating the stainless steel with teflon had little effect on the stress/strain response of the rod in solid oxygen. The data from Table 1 are plotted in Fig. 5.
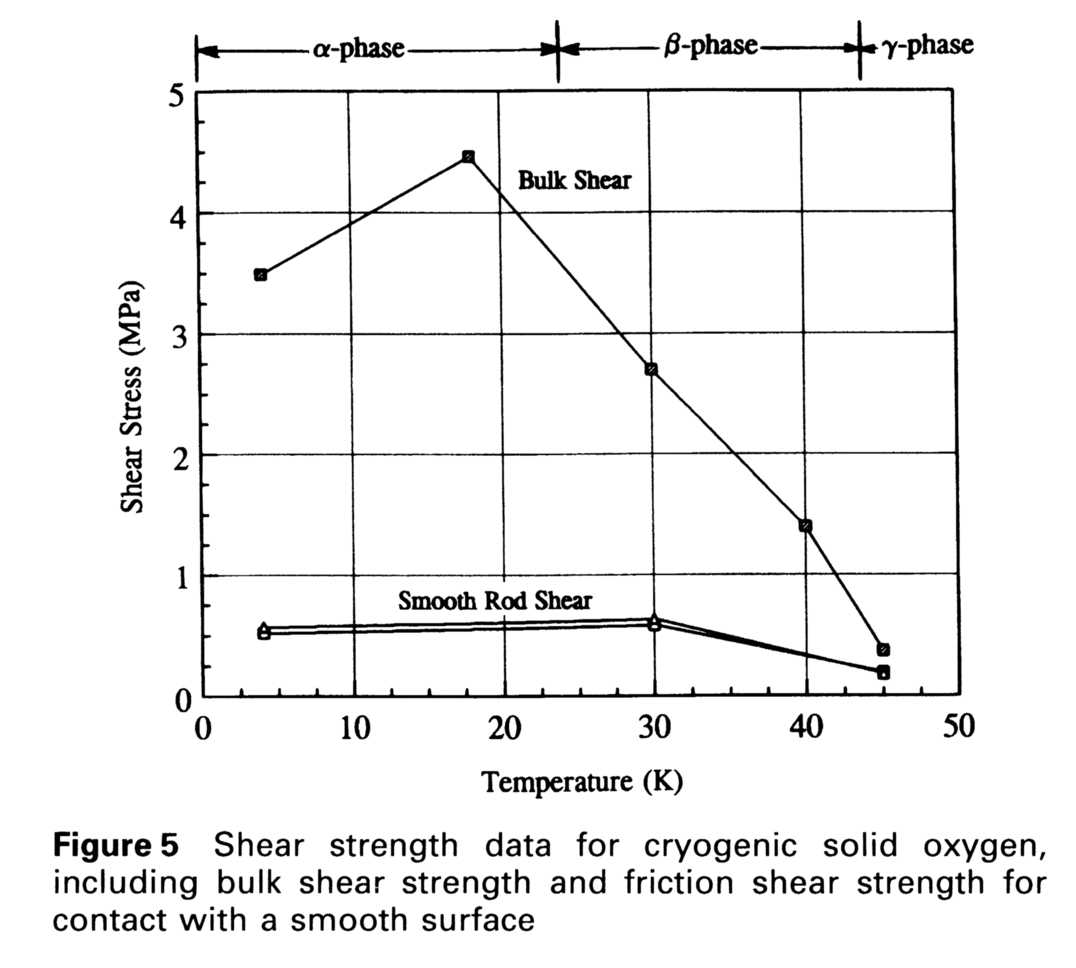
The stainless steel and teflon results were very similar in magnitude and response to displacement, and the shear rod tests were also consistent with these results, leading to good overall confidence in the data. The shearing apparatus had a peak load capacity of about 9 kN with a safety factor of 3. The resolution of the force measurement was determined by the accuracy of the load detection: 0.2% (about 5 N). The resolution of the shear measurement is thus better than a few percent based on a shear area of 10 cm2. The error in the shear stresses calculated may be large (a significant fraction of the data itself), however, because of a lack of knowledge of the details of the shearing and thus the area used to calculate stress, which also affects the load offsets for the smooth rod shearing.
DISCUSSION
The bulk shear results given above have been calculated using only the groove area at the outer diameter of the bolt where the shearing takes place, and excluding the effects of adhesion at the smooth large diameter sections of the bolt except for the 45 K case. Initial results (7) were calculated subtracting a computed load from the adhesive strength of the solid oxygen in contact with the outer diameter of the bolt by using the smooth rod stress data. Reexamination of the details of the strain behavior has shown this to be incorrect. The stress vs. elongation breakaway behavior of the solid oxygen from the smooth bolt is shown in Figure 2. It indicates an elongation varying from 0.11 to 0.13 cm at breakaway and a very low strength after breakaway. Examination of the stress vs. elongation results shown in Figure 4 for the grooved rod indicate that for the data at every temperature below 45 K there is a drop in load at precisely the elongation corresponding to the breakaway elongation for the smooth rod tests. This indicates that the solid oxygen has torn away from the outer bolt surface before the peak load has been reached and therefore does not contribute to the bulk shear strength measurement except at 45 K. This behavior also enhances the reliability and accuracy of the bulk shear strength data at temperatures below 45 K since uncertainties in the stress area are reduced.
It should be reemphasized, however, that the experimental configuration that was used provides only an approximate measurement of bulk shear strength. A reference material of known strength was not tested to verify the accuracy of the measurement technique. A number of general inferences may be drawn from the data, however. The trend of decreasing strength and increasing plasticity with increasing temperature is a characteristic of all materials near their melting point. Most pure materials, however, do exhibit constant strength over a wide temperature range, which does not appear to be the case with solid oxygen. Examination of the details of the strength behavior of solid oxygen is complicated by the effects of its solid phase transition, and the unusual properties of the phases themselves.
Each crystal phase of solid oxygen has distinctly different properties. The γ phase recrystallizes in a few minutes (10) such that most experimental work on the γ phase solid is done on single or large crystalline samples. Although the linear expansion of the γ phase is large (780 x 10-6/K (10)) internal strain is rapidly accommodated by recrystallization. The γ to β phase change is accompanied by a 5% volume change and a change from the orientationally disordered γ phase to a parallel orientation of the oxygen molecules in the β phase (11). Together, these changes cause randomly oriented fine-grained crystals of the β phase to form throughout the solid (10). The γ crystal is soft and transparent, whereas the β crystal is hard and opaque (10). Warde and Wheeler describe the phase transformation as fracturing the crystal and making it translucent (13). The β crystal is unusual because of its lattice behavior; the a axis has double the expansion rate of the γ phase, whereas the c axis has a slightly negative expansion rate (10). The β crfstal can be annealed overnight at 31 K, where no grain growth was observed to occur (10). The β to α phase change, however, is accompanied by a small volume change (12) and no major grain size change or orientation change. Numerous authors (e.g. 6,13) refer to the extreme difficulty of obtaining large crystals of the β phase and to date none have produced large crystals of the C solid. The structure of the α phase has been carefully studied, however, and no change in the lattice parameters of the α phase was observed in the temperature range from 23 to 4 K (12). The α phase is notable for the simple nature and small magnitude of its twinning shear (12). The molecular structure of the α phase of solid oxygen causes it to be the only known molecular antiferromagnet (3).
These details have various affects on the interpretation of the shear strength data discussed above. The γ phase data is expected to be affected the least by the details of the behavior of solid oxygen. Large, annealed, isotropic and orientationally disordered γ phase crystals of solid oxygen should lead to simple macroscopic behavior and relatively weak crystals, consistent with the data described here.
The γ to β phase change should result in very different properties of the β phase. The hardness change between γ to β phase change should be seen in shear strength testing but is not obvious from the shear data of Fig. 5, probably as a result of the single γ phase data point. The duration of the experiments described above imply that the β crystals were polycrystalline and probably not annealed, possessing significant internal strain. Barret and Meyer (12) observed grain growth of the β solid during an overnight anneal at 42 K. The samples tested in this work were held at temperature for at most an hour by the time of testing. In any case, the shear tests show that solid oxygen is not a strong material; the internal strains generated by the contraction of the phase change and cooling should cause plastic deformation of the solid to the extent that the internal stress is equal to the yield strength of the material on short time scales after temperature changes. The β crystal grains that are randomly oriented, and each grain undergoes large contraction during cooling of one axis, so, aside from the phase change contraction, and the crystal grains should be strained to their limit for hour long time scales before annealing or recrystallization can relieve these strains, very probably at 40 K and almost certainly at 30 K. The highly strained nature of the β to α phases seems to be a description of the crystals tested here. This, in turn, would indicate that the unstrained bulk shear strength of both of these phases is somewhat greater than that measured here.
The increase in strength with decrease in temperature of solid oxygen, down to 18 K is consistent with decreasing crystal molecular and defect mobility with decreasing temperature. The multiple load drops observed in the 4 K test of α oxygen can be identified as twinning of the crystal as discussed above. This type of behavior has been observed in iron mechanically deformed in compression at 20 K and 4 K (14,15).
The smooth bolt shearing tests must also be strongly affected by the relatively large residual internal stresses in the crystal relative to strength of the β and α phases. The measured shearing forces were found to be nearly identical for the sanded stainless steel surface and the teflon coated surface. Both surfaces possess surface area features that are on the scale of 0.1 mm with a typical depth somewhat less than that. The chemical nature of the surface changes dramatically between stainless steel and teflon but this change probably does not change the shear strength test results because the oxygen should bond to both materials, unlike most room temperature materials. Stress on the smooth bolt surface is also biaxial as a result of the compression that is caused by the contraction of the γ to β phase change and the cooling of β crystals. The area, friction, and total stress on the true shearing surface are certainly hard to predict. The fact that the measured shear strength is almost the same at 30 K and 4 K implies that the bulk strength of the solid oxygen is probably not the controlling factor, since the grooved bolt shearing tests imply a strength increase of almost a factor of 2. Although the compressive stress increases as the β crystal cools from 30 to 23 K and its yield strength increases, the α phase undergoes only a small further thermal contraction at the phase change and none caused by cooling to 4 K. However, if the solid strength is greater than the interface strength between the oxygen and the bolt, the stress measurement would depend on that strength, which probably does not change with temperature because the chemical bonding strength would not be expected to change between 30 K and 4 K. Furthermore, with an uneven surface, many small voids might form after small displacements, reducing the true shearing area dramatically, such that the actual shearing strength is much higher than that derived from a straightforward calculation because the true shearing area is much lower than that assumed. In any case definite conclusions about these results cannot be made without further testing. A coincidental benefit of the similar smooth rod shear force results with and without the teflon coating is that the similarity demonstrates the repeatability of the oxygen shear testing; the peak loads and the shape of the curves at the three temperatures were practically identical for the coated and uncoated bolt tests. The smooth rod tests do not represent the true strength of solid oxygen, but they do demonstrate an important property of the solid for practical engineering work.
CONCLUSIONS
Experiments were performed that have provided fundamental new data on the strength of solid oxygen as a function of temperature. An apparatus and procedures for strength testing of cryogenic Van der Waal solids were developed to overcome difficult experimental problems. Solid oxygen was found to behave as a plastic material with a shear strength of approximately 0.3 MPa 10 K below its melting point. Solid oxygen becomes increasingly stronger and brittle as its temperature is decreased, giving a shear strength of over 3.5 MPa at low temperatures. More careful experimentation is needed to explore the mechanical strength and adhesive behavior of all of the phases of solid oxygen.
ACKNOWLEDGEMENTS
This work was performed at Advanced Materials Laboratory, Inc. through funding from an SBIR program by the Air Force Phillips Laboratory under contract # F29601-92-C-0094.
REFERENCES
a. Bates, S.C., and Altshuler, T.L., "Shear strength testing of solid oxygen," Cryogenics, 35, #9, (1995) 559-566.
1. Roder, H. M., "The Molar Volume (Density) of Solid Oxygen in Equilibrium with Vapor," J. Phys. Chem., (1978), 7, #3, 949-57.
2. English, C. A. Venables, J. A. "The structure of diatomic solids" Proc. R. Soc. Lond. A., (1974), 340, 57-80.
3. Etters, R. D. Helmy, A. A. and Kobashi, K. "Prediction of structures and magnetic orientations in solid alpha and beta-O2," Phys. Rev. B-Solid State2 3rd Series, Aug. 15 (1983), 28, 2166-2171.
4. Ahern, J. E., and Lawson, T. W., "Cryogenic Solid Oxygen Storage and Sublimation Investigation," Aerojet General Report No. AMRL-TR-68-105, NTIS Document # AD687852, (1968).
5. Voth, R. O., and Ludtke P. R., "Producing slush oxygen with an Auger and Measuring the Storage Characteristics of Slush Hydrogen," NBS Report No. NBSIR 79-1607, March (1979).
6. DeFotis, G., "Magnetism of solid oxygen," Phys. Rev. B, (1981), 23, #9, 1 May, 4714-4740.
7. Bates, S. C., "Discrete Injection and Storage of Solid Oxygen," SBIR Phase I Final Report USAF Phillips Lab Contract # F29601-92-C-0094 Report # PL-TR-93-3013, (1993).
8. Altshuler, T. L., "Behavior of Metal Matrix Composites at Cryogenic Temperatures," Naval Surface Warfare Center Report NSWC TR 87-216, 29 April (1987).
9. Scott, R. B., Cryogenic Engineering, Met-Chem Research Boulder CO (1959).
10. Barrett, C. S., Meyer, L., and Wasserman, J., "Expansion Coefficients and Transformation Characteristics of Solid Oxygen," Phys. Rev., Nov. 15 (1967), 163, #3, 851-854.
11. Kobashi, K., and Klein, M. L., "Lattice dynamics of solid oxygen," J. Chem. Phys., 15 July (1979), 71, #2, 843-849.
12. Barrett, C. S. Meyer, L., "Molecular Packing, Defects, and Transformations in Solid Oxygen," Phys. Rev. Aug. 15 (1967) 163 #3 694-697.
13. Warde, C., and Wheeler, R. G., "Optical and structural properties of solid oxygen films," J. Phys. C:Solid State Phys.(1978) 11 1717-1732.
14. Altshuler, T. L., and Christian, J. W., Philosophical Transactions of the Royal Society of London Series A., 8 June (1967), 261, #1121, 253-287.
15. Altshuler, T. L., and Christian, J. W., Acta Metallurgica, (1966), 14, #7, 903-908.