Elevated Temperature Oxygen Index Apparatus and Measurements*
Dr. Peter R. Solomon
Advanced Fuel Research, Inc.
Abstract
An apparatus that performs elevated temperature (300-800°C) Oxygen Index (OI) measurements using radiant heating has been developed and used to perform OI measurements on a variety of high-Ol materials. Comparisons of data with OI values obtained using a gas preheat device indicate reasonable agreement. Flame propagation down the edges of the samples dominated the burning behavior for many high temperature samples, resulting in OI measurements not indicative of most actual fire situations. The physical mechanism of volatile release was identified as the controlling factor for the OI value for high-Ol materials. Delamination, cracking, matrix penetration, foaming, and blistering all led to different burning behavior and different OI characteristics. Long-term aging of composite materials is also a very important contributor to the OI of the material. At temperatures above 300°C OI values were found to be very high as a result of the stable nature of the carbon char remaining after decomposition of the polymeric binder material.
*Published in Journal of Fire Sciences, Vol. 11, p. 271 (1993).
Introduction - Polymers and composites are being steadily improved, as their military applications increase rapidly. In most cases the materials must pass a variety of tests [1,2] that range from flammability and smoke release, to effluent toxicity. One widely used quantitative relative ranking of fire resistance is provided by the oxygen index (OI). OI tests were originally developed at room temperature and have ASTIVI standards to make testing uniform [3]. For advanced materials, testing at elevated temperatures is more relevant for evaluating fire susceptibility. Tests by Macaione [4] for the Army and others [5] show that OI values can vary dramatically with temperature, usually decreasing as the temperature increases, and often changing the relative ranking of some materials. As better materials withstand steadily harsher conditions, the Army has found its needs for high temperature testing surpassing the capabilities of currently available instruments. High temperature OI standards have recently been developed for the Navy [6], and commercial standards will likely follow.
At present the OI test is the predominant test method used for judging material flammability by chemists engaged in developing new materials. The test is very influential because it is simple, easy to calibrate, and requires only a few grams of test material. The test measures the minimum ambient oxygen concentration needed to support a creeping flame spreading down a small material sample. Fire researchers have long questioned the significance of the OI test, or indeed any flammability test whose results are not confirmed by full-scale fire tests; there is little correspondence of material hazard rankings between the OI test and full-scale corner tests which evaluate resistance to flashover. For example, polyvinyl chloride plastic is particularly hazardous in larger-scale fires but performs well in the OI test. The reason for this discrepancy is that gas-phase fire retardants, such as halogens, act by reducing gas-phase chemical reaction rates. These retardants are particularly effective against the small flames in an OI test, which provides only a brief flow-time for release of heat, but are much less effective against larger hazardous-scale fires whose longer flow-times allow reactions to go further toward completion and release of heat.
Oxygen Index Apparatus - The apparatus that was assembled to perform elevated temperature OI measurements is shown in Fig. 1. The sample chamber is 450 mm long and 80 mm wide with a constriction at the top, consistent with the specifications for room temperature OI testing as described in ASTM standard D 2863-87. The chamber is square, built out of sheet steel and sealed with high-temperature silicone adhesive. It has long windows on two sides to admit the radiant heating and another window on a third side to permit flame viewing orthogonal to the radiation. Samples are mounted on a rod that extends through an O-ring seal at the bottom of the device to a traversing mechanism and drive motor. The traverse mechanism translates the sample at a rate that is variable from 0 to 1.4 cm/min over 6 cm. This compensates for the typical 1 cm/min propagation rate of the flame down the sample at the OI, allowing the radiant heating zone to remain at a constant position relative to the flame.
Pressure-regulated pure gas cylinders supply oxygen and nitrogen flows, metered separately by needle valves for precision and calibrated against rotameters. The calibration provided greater precision than could be achieved with the rotameters, although the rotameters allowed faster OI testing and were used exclusively for later tests. Since the rotameters were inaccurate for very low flow rates, the apparatus was limited to 10-90% oxygen, preventing measurements at the extremely high OI's of some of the advanced materials tested. The two gases are joined and undergo complete turbulent mixing before they reach the flow chamber. The high velocity jet of mixed gas enters a manifold through a screen that breaks up the jet, and fills a flow distribution manifold in the bottom of the sample chamber that is created by a large pressure drop filter. The chamber is carefully sealed below the exhaust contraction at the top to prevent the entry of room air that would otherwise be sucked in by the lower internal pressure of the buoyant heated gas. The gas flow rate is specified as 4 ± 1 mm/sec at 0°C and atmospheric pressure; the OI is insensitive to flow rate [7].
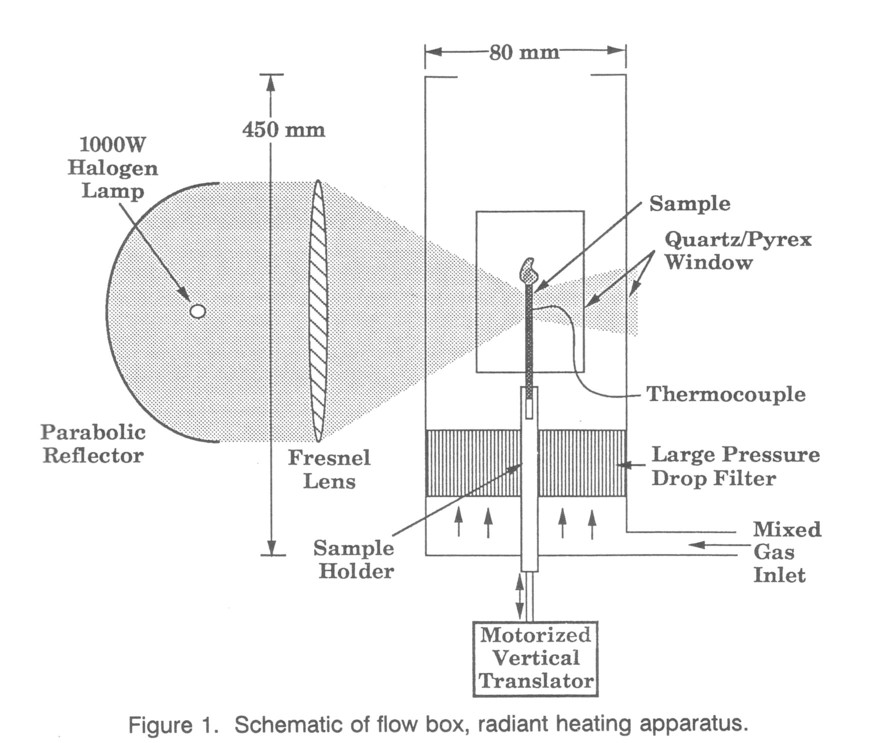
Radiant Sample Heating - Achieving a high and uniform sample temperature with a minimum delay and a moderate use of energy is the goal of radiant heating. The commercially available Stanton - Redcroft device (practical OI measurements to 300°C) uses one set of electric coils to preheat the gas by conduction and another to provide additional heat to the sample through infrared radiation. At temperatures above 400°C electric power requirements for this become prohibitive, and radiant heat losses increase dramatically. The advantage of high-temperature lamps over lower temperature furnace coils is that the radiant power is proportional to T4 and much more power is emitted for a given electrical input. Furthermore, the energy expense and difficulty of heating the gas is avoided. Gas preheat is not necessary for OI testing because 1) heat transfer between the gas and the sample is negligible during radiant heating, and 2) gas temperature (and density) does not significantly affect the flame temperature; only the oxygen concentration is important.
The radiant heating apparatus used for this preliminary apparatus was designed for maximum radiant power at minimum low cost. The apparatus consists of a 1000 W halogen lamp, a 46 cm diameter spun aluminum parabolic reflector, and a 34 cm diameter acrylic fresnel lens. The lamp was placed at the focus of the reflector, resulting in a large collimated beam, which is focussed on the sample by the fresnel lens. The optics must have a dimension large compared with the 2 cm long lamp filament to prevent defocussing at the sample caused by size of the light source. Filter goggles must be worn during setup at all but minimum lamp settings, but direct flame inspection can be done for OI determination because the lamps are shielded by the light-gathering mirrors.
This system has a high f-number compared with more expensive elliptical mirrors. Losses result from a limited collection solid angle, reflection losses, lens losses, and spectral losses. Since the parabaloid focus is 5mm in front of the mirror, slightly less than half of the radiation emitted from the lamp is collected. The mirror is spun aluminum, so that its surface is only 80-90% reflecting, and has undulations that defocus the image. The fresnel lens (the largest inexpensive lens available) has a focusing aperture 34 cm in diameter, only focussing 55% of the mirror beam. Also, since the lens is plastic, much of the infrared radiation is absorbed and lost, although this only accounts for 15% of the total lamp radiation. Future experiments will use radiant power meters to quantify the delivered radiant heating.
Apparatus performance tests - OI measurements were performed for sample temperatures up to at the maximum lamp power rating, without damage to the equipment. An OI result of 17.5 for plexiglas at room temperature was obtained, matching literature values and verifying the oxygen-nitrogen mixing and calibration, and the overall performance of the apparatus. A thermocouple inside the OI chamber (to minimize distortion by buoyant flows) was traversed across the lamp image in free space to show that the horizontal heating was uniform and wider than the sample. Vertical temperature profiles taken by a thermocouple on the sample surface showed that at all flux levels there was a zone of uniform temperature that was broad compared with the flame width of a few millimeters. At low flux levels and lower sample temperatures time to equilibrium is long, but at high flux levels equilibration takes place in a few minutes.
A small elliptical mirror with an f-number of 0.9 was also tested for radiant power transfer. This mirror is electroformed and relatively inexpensive, but captures a large solid angle and has a very high reflectance across the lamp radiative spectrum. For comparison, the mirror was focussed on a five cm square, 6 mm thick piece of poco graphite. Using a 300 W lamp this mirror heated the graphite to the same temperatures as one of the larger fixtures described above that used a 1000 W lamp, indicating an efficiency gain of approximately a factor of 3. All three sources together were able to heat the graphite to 800°C in air as measured by an uncorrected thermocouple probe (actual temperatures are about 50°C higher). Actual sample temperature heating capabilities are much higher, since the graphite has a much higher thermal conductivity than composite plastics, and convection cooling is reduced in the OI apparatus.
Oxygen Index Testing - The materials that are currently used for military applications must survive extreme conditions, and thus usually have high OI values. These materials have unusual polymer formulations for maximum thermal stability, and are often used in combination with a less combustible matrix as a composite. Fire retardants and fire retardant fillers such as graphite are also often added. When subjected to heating and radiant flux, a material undergoes a variety of changes before it losses its functional integrity. Changes in radiative absorption, physical shape, internal chemical structure (curing, secondary reaction), and release of loosely bound volatiles all may cause changes in the fire resistance of the material.
Specifics of the OI test itself strongly affect OI values. The OI is defined as the oxygen concentration for which the sample burns for exactly 3 minutes. Termination of burning is defined as the time at which there is no remaining visible flame. This is a somewhat subjective criteria that is often made more definite by an associated puff of smoke. The OI is also determined by reaching an oxygen concentration threshold where the flame burns down a distance greater than 5 cm. This criterion applies to materials that exhibit no significant burning until the Of is reached, at which point the flame burns down the edge rapidly. Glowing combustion after flame extinction must be noted, together with its duration. At elevated temperature the question of sample thermal equilibrium certainly becomes important, but has not been extensively studied. The sample should not exhibit any signs of decomposition for simple measurements. Release of smoke, change in color, or change in the appearance of the surface indicate that changes in the sample may distort the OI measurement. The sample must be ignited over the entire top by a small external flame. The time required for ignition will vary with material type and OI, but care must be taken not to apply the external flame for a period longer than that required to ignite the sample.
Major changes in OI behavior arise from whether the entire sample vaporizes and burns, or whether there is a residual matrix. A further difference in burning behavior occurs if the matrix is left as a char or an inert material, such as with fiberglass or metal composites. The matrix affects burning in a variety of ways. Above the flame it functions as a heat sink and interferes with the formation of the flame itself. Beneath and below the flame it affects the OI measurement by determining the path for the escape of the volatiles from the bulk material to feed the flame and the condition of the material. Farther down the sample it affects the heat transfer gradient that is important for the OI test. For instance, the low thermal diffusivity of fiberglass steepens the vertical temperature gradient, causing less heat to be required for the initial heating of the sample.
There are also physical changes in the sample itself that affect heat transfer and volatile release. Some materials swell and crack, warp, or foam in response to localized heating. Foaming or swelling greatly decreases heat transfer to the inside of the material, while cracking provides a much shorter path for the release of volatiles, increasing the rate of devolatilization locally around the crack. One mechanism that can enhance burning is the slow, glowing combustion of the char, which forms a steady heat source, while the varying volatile release might otherwise lead to extinction of the flame. Another phenomena that is very typical of OI testing of fire resistant materials is preferential edge burning.
Advanced materials exhibit many different behaviors that are not seen in common materials, but affect the OI test. Care must be taken that the test be done in a manner truly representative of the application of the material. For a particular composite this might mean that edge burning must be avoided in the OI test because the material delaminates easily. Delamination would decrease the OI in a standard test, but increase it in a real applications because the material was molded and bordered such than there were no exposed edges to burn, so that delamination would reduce internal heat transfer and reduce volatile release.
Five materials were chosen for extensive OI testing on the basis of their high OI values and availability (Table 1). Three were commercial and two were from a set of samples supplied by Dr. Domenic Macaione of the Army Materials Technology Laboratory (MTL). Mass loss vs. temperature was measured by thermogravimetric analysis (TGA), and the physical characteristics of the decomposition of the material were observed and catalogued. The TGA measurements used a heating rate of 30°C/min, which may be too fast for some samples, indicating evolution temperatures that are slightly higher than the actual temperatures because of slow decomposition rates.
Table 1. OI Testing Materials
Trade Name.....................Material..................Supplier
..G-10.......................Epoxy/glass...............Westinghouse
Ryton-4 06............Polyphenylene Sulfide...............Phillips
Black Phenolic...............Phenolic...................Westinghouse
Ferro-6P.................Polyester/glass..................Army MTL
P-14020......................Phenolic.....................Army MTL
Testing was performed from room temperature up to the limit of the preliminary radiant heating apparatus, approximately 550°C. The practical temperature limit on OI measurements was approximately 300°C because sample decomposition prevented an equilibrium sample temperature from being achieved at constant radiant flux. Typically, heat transfer to the bulk sample becomes unstable under radiant surface flux as the material either changes adsorption (color) or the surface separates from the bulk (delamination, foaming, etc.). This limitation can be overcome by controlling the radiant flux with surface temperature feedback.
OI measurements for G-10, Ryton-4 06, and Black Phenolic are shown in Fig. 2. Testing of these materials will be described in the order that they are listed in the table. A limitation in interpreting results arises from the lack of detailed knowledge of the chemical composition of the samples.
The high OI values for G-10 were typical of the fiberglass samples that were tested. Also typical was the slight but steady decrease in OI with temperature as less heat is needed to sustain devolatilization and burning. Like many of these materials, the oxygen index was reached when sustained burning was achieved down one edge of the sample. Vapor was driven out between the fiberglass layers and ignited as jets. Slightly below the OI the top of the sample would burn well, but the flame would go out before propagating down the sample. At the OI burning proceeded rapidly down one edge. The transition in oxygen concentration is very sharp between short burn-time and rapid burning, as opposed to many other materials which burn for a gradually longer time as the oxygen concentration is increased.
G-10 was also used to take OI measurements at the high-temperature limit of the apparatus because it maintained structural integrity at these temperatures. The sample was baked out below the flame zone, and the lamps turned to maximum intensity, requiring the windows to be air-cooled Sample surface temperature was measured as 550°C. At these temperatures the sample could not be lit at the highest measurable oxygen concentration an 90%), because only the glass matrix was left in the sample.
G-10 has a smooth, shiny, translucent green surface, such that radiation absorption occurs throughout the bulk sample and the center heats fastest At 210°C the epoxy begins to degrade, turning a light brown. As to temperature increases the material darkens progressively and delamination begins, seen as successive darkening spots that grow to the width of Me sample in a few seconds (very quickly) as each internal layer heats up. As the surface temperature increases beyond 330°C its color has turned black, and bubbling liquid is driven to the surface through the fiberglass weave. The sample temperature stays constant while all the liquid boils off into copious smoke and then it rises continuously again. TGA measurements indicate that the beginning of the devolatilization can occurs close to 330°C as is observed with the radiant heating, while the mass loss beginning at 400°C is the burr of the epoxy char.
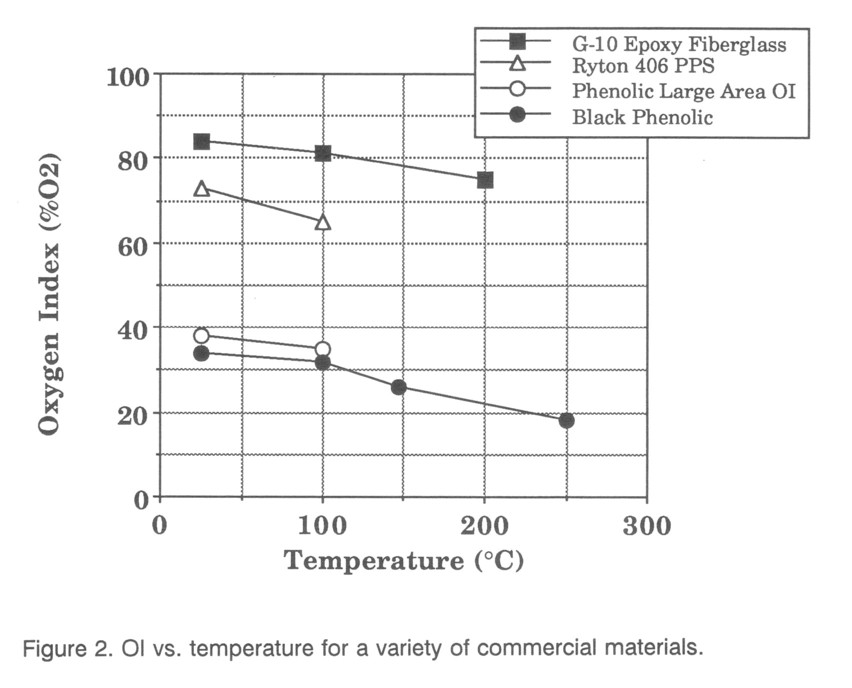
The OI values for Ryton-4 06 are also shown in Fig. 2, and are quite high. This smooth and shiny black material is described as glass filled and has a heating behavior dominating by bubbles and foaming. Bubbles begin to form near 200°C and the bubbling surface expands at higher temperatures. The surface tension of the liquid is such that the bubbles formed by the vaporizing component of the plastic do not burst, but form an expanded, fairly strong, surface that is then in extremely poor thermal contact with the bulk material beneath. This is an excellent fire prevention property, but it prevents radiant heating from achieving uniform sample heating. At about 340°C visible smoke forms, becoming denser as temperatures increase. TGA data to Ryton-4 06 in air show a small but steady mass loss beginning at 350°C, and significant mass loss caused by pyrolysis beginning near 500°C. The TGA results in nitrogen show only the pyrolysis devolatilization and thus identity a second mass loss in oxygen at 650°C as burning of the char.
OI values vs. temperature for the black phenolic are shown in Fig. 2. This material is typical of another class of materials where the matrix can exhibit glowing combustion without a flame. The OI is determined as usual by the existence of a flame for 3 minutes after ignition, with an addendum about the persistence of glowing combustion. This material also shrinks during burning, and the volatiles that feed the flame are released primarily from the crevices. This behavior leads to non-steady burning, usually down an edge, and the glowing combustion can lead to reestablishment of a flame when it would otherwise appear to have gone out.
The black, semi-gloss phenolic also begins to decompose near 200°C, when small blisters (≤ 1 mm diameter) form. At temperatures above 250°C, larger blisters form as the outside layer delaminates, preventing stable temperatures from being reached. The surface is brittle and cracks open such that high temperature OI measurements are dominated by the time these cracks are able to supply fuel to a flame; burning time varies greatly from test to test. TGA curves in air and in nitrogen indicate that there is a significant amount of water in the samples, and that primary devolatilization begins at 300°C, while secondary devolatilization begins at 450°C after the material has pyrolized. TGA tests in air imply pyrolysis followed by combustion at higher temperatures, with the matrix of paper burning away almost completely.
All of the samples tested extensively exhibited preferential edge burning to some degree during testing, in the case of G-10 burning down one side, or in the case of black phenolic burning preferentially toward one side but not at the edge. This behavior suggests that a surface burning test without edges would give higher OI values, and might be a more representative measurement if, as in most applications, the edges are covered. Edge burning is a common phenomenon and can be inhibited by smoothing or sealing the edges. This changes the heat transfer near the edges and the global burning.
As one solution, 5 cm (vs. 1.2 cm) wide samples were put into the OI apparatus and the center of these samples lighted. Burning proceeded vertically down the center of the sample rather than out to the edge, forming an excellent surface-burning OI test. OI values were somewhat higher than the standard values, as expected. Both the G-10 and black phenolic materials were tested in this way. G-10 surface OI values were above the testing limits of ≈ 90% 02. Black phenolic surface OI values are shown in Fig. 2. The amount of increase in OI will depend on the ease with which edge burning occurs for a particular type of sample. A surface burning of plexiglas was done to demonstrate that this surface burning would occur in a similar manner with a non-composite material. As was expected, the OI remained the same in surface burning tests, since plexiglas is not an edge burning material. The surface-burning test seems to be a superior OI measurement for normally edge-burning materials.
Ferro-6P is a textured, shiny white fiberglass composite woven in a mat with 5 mm wide strips for the laminate. Its OI values as measured by the Army MTL show an unusual temperature dependence. OI values increase dramatically above room temperature to very high values. Tests shown in comparison with the Army data are given in Fig. 3. Once again the change in color prevented a stable high temperature measurement. Values at 25 and 200°C agreed closely, while those at 100°C differed significantly. The discrepancy was resolved by first baking samples at 100°C and then testing them at 25°C, giving the high values identified with the higher temperatures. The cause could be curing of the polymer or vaporization of a loosely bound component. In any case the low room temperature OI value is definitely dependent on the history of the material. The decomposition behavior shows no major second high temperature weight loss resulting from char burning.
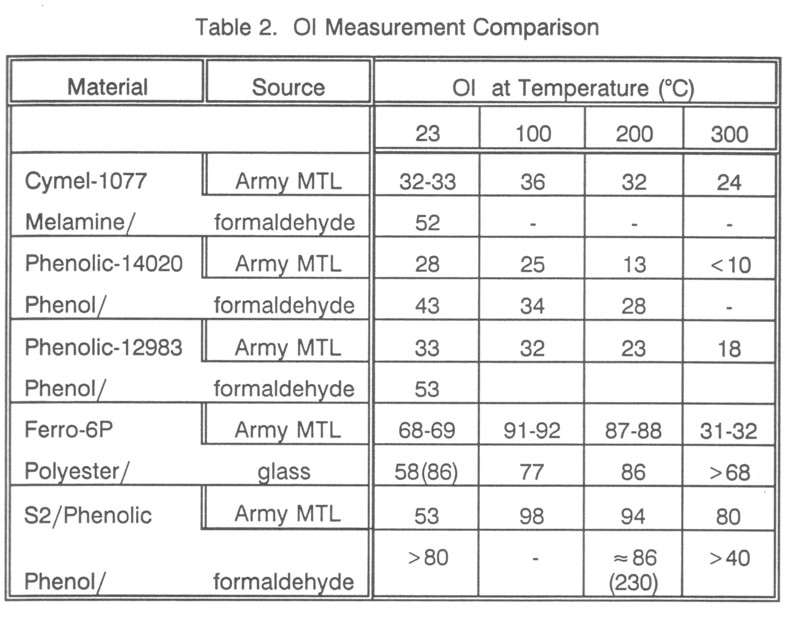
To further investigate changes in the Army samples, P-14020 phenolic was tested. The Army MTL OI data for this material (Table 2) shows more typical OI behavior. Radiant heating OI tests indicated significantly higher values, although the same general temperature dependence. The probable cause of this difference is that the Army MTL data was taken for fresh samples, which had aged for years before testing in this program. Presumably slow room temperature devolatilization took place in the materials, raising the OI.
Given the changes discovered in Ferro-6P and P-14020, it was decided to test other sample materials from the Army Materials Technology Laboratory (Table 2). These materials were intended to provide some comparisons of OI data between the radiant heating apparatus and the standard Stanton-Redcroft apparatus. Some were tested at room temperature to eliminate the possibility of differences in OI values caused by the technique of radiant heating. The OI values are seen to be significantly higher in all cases, except for some of the higher temperature cases where one would not expect the long-term aging behavior to be important.
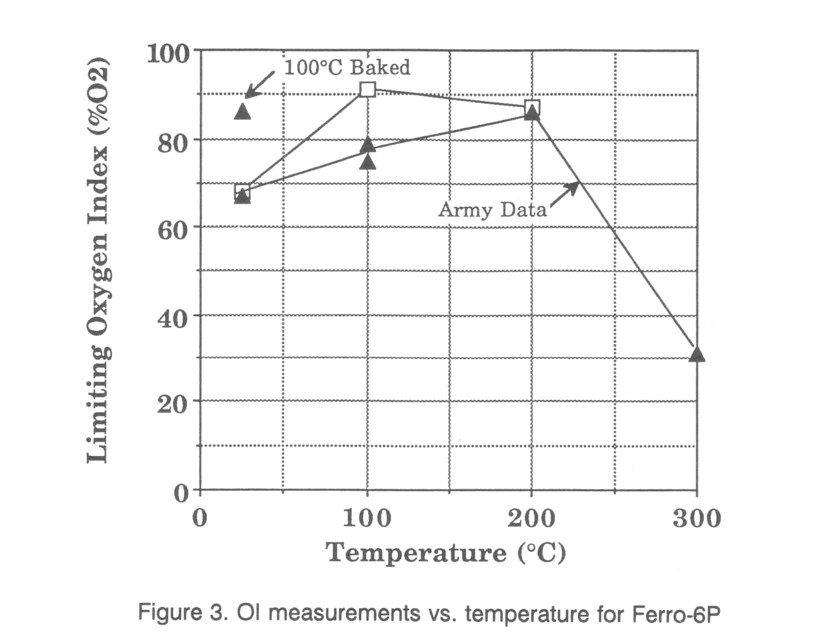
The testing in the radiant heating apparatus also indicates the equilibration of sample temperature should be investigated. The radiant fluxes provide a much greater heating rate than that obtained in the standard apparatus, so one would expect a faster equilibration. Sample heating tests at 300°C indicated that significant decomposition was in progress. This would distort OI values in the preheat apparatus because decomposition cooling would lower the internal temperature compared with the measured surface temperature. Some indication of this is seen by the 100°C OI values for Ferro-6P, which lie between the room temperature measurement and the Army MTL measurement, presumably because the process changing the sample was still taking place.
During OI testing it became clear that radiant heating at high temperatures interacted more with the sample than does preheat, through changes in the absorptivity of the sample as the material decomposes. In general, radiant flux provides a means of sample heating that may be more indicative of actual fire conditions because fire spread is caused primarily by radiative heating from the flame near the material. Data was taken for the G10 epoxy fiberglass and the black phenolic, indicating a similar decrease in OI with flux as is seen with temperature.
Conclusions - A preliminary apparatus for OI testing at -elevated temperatures using radiant sample heating has been developed and used to perform OI testing of fire resistant materials. OI measurements are based on both sample temperature and radiant flux. Uniform radiant sample heating was performed to over 550°C, and 800°C temperatures were achieved with a demonstration apparatus that showed that such temperatures could easily be attained in a next generation device. Comparisons with plexiglas OI values and Army OI data verified device performance and procedures. Testing demonstrated that current techniques could be significantly improved without significantly altering test procedures by: (1) Testing using center ignition with samples to avoid edge-dominated burning of composite materials, (2) Testing based on radiant flux as well as sample temperature, and (3) Measurements performed on sample equilibration.
The physical mechanism of volatile release was identified as a prime determinant of the OI value for high-Ol materials. Delamination, cracking matrix penetration, foaming, and blistering all led to different burning behavior and different OI characteristics. Edge burning was identified as a dominant burning mechanism for many high temperature samples, resulting in OI measurements not indicative of most burning situations. Central ignition of wider samples lead to surface-burning OI values 3-5 points higher than #0 edge dominated burning results. Surface emissivity changes with temperature greatly modify the absorption of radiant energy and heat flux to the sample. causing important changes in the material response to fire conditions. This phenomenon is measured by performing OI comparisons at constant flux.
Comparison of OI measurements with Army data show that for composite materials long-term aging and curing at temperature are very important factors in determining the OI of the material. OI measurements of most of the materials tested were limited to approximately 300°C by decomposition of the polymeric binder material under radiant flux. At temperatures above this, OI values were found to be very high as a result of the stable nature of the remaining carbon char.
Acknowledgement - The authors thank the U.S. DOD, Army MTL for funding this work under Contract # DAAL04-91 -C-00I7. Special thanks to Dr. D. Macaione for extensive discussions and for providing samples for tests.
REFERENCES
1. Nelson, G.L., Editor, ACS Symposium Series # 425, Amer. Chem. Soc., Washington, DC, (1990).
2. Drysdale, D., John Wiley and Sons, New York, NY (1985).
3. ASTM Standards D 2863-87, Annual Book of ASTM Standards, (1989).
4. Macaione, D.P., Dowling, R.P., 11., and Bergquist, P.R... Summary Report AMMRC TR 83-53, Army Matl. & Mechanics Research Center, Watertown, MA, (1983).
5. Redfern, J.P., Tech. Inf. Sheet No. 24, Stanton Redcroft, Surrey England.
6. Mil. Standard 2031.
7. Werley, B.L., Editor, ASTM STP 812, American Society for Testing and Materials, Baltimore, MD (1983).