HIGH TEMPERATURE TRANSPARENT FURNACE DEVELOPMENT
Kim S. Knight
40 Nutmeg Lane
Glastonbury, CT 06033
PROJECT SUMMARY
The purpose of this research was to design, fabricate, test, and deliver a transparent furnace that operates at temperatures up to 1200°C. Full optical access to high temperature furnace applications is needed to diagnose the subtleties of many processes. Radiation containment and convection elimination are used to reduce electrical power consumption and component heat loads. The process chamber in the furnace can operate with any gas at pressures between full vacuum and one atmosphere.
A heat transfer model has been developed to predict the behavior of the transparent furnace and permit projection of performance improvements that occur as a result of modifications in furnace design. Axial and radial heat losses through conduction, convection, and radiation were predicted as a function of temperature for different furnace configurations.
A variety of techniques have been investigated reduce heat losses compared with lower temperature transparent furnaces. The major improvement has resulted from evacuation the space between the process chamber and the outer furnace tube to eliminate convective heat transfer. High vacuum was found to be necessary to eliminate convection; even 0.001% of an atmosphere pressure led to large convective heat losses. The infrared radiation reflective coating was improved, eliminating most of the radiation absorption in the outer quartz shell. Radiation shields were added to the ends of the furnace to further reduce radiative heat losses. Conductive heat losses were minimized by minimizing solid connections to the cooled furnace end caps and using quartz components. Components were designed to survive high temperature operation. Extensive experiments were performed with a succession of preliminary prototypes to explore and demonstrate furnace improvements.
The final prototype was tested at 1200°C, as was the computer control and data acquisition software, the vacuum system, and the electrical systems. A graphical monitoring and control system was developed to operate the furnace. A user's manual was written and the prototype transparent furnace was delivered to NASA.
A high temperature transparent furnace will create many new opportunities for high temperature material processing research and process control applications. Crucial applications that only take place at these high temperatures include melting copper and growth of a number of important II-VI material crystals.
2. EXECUTIVE SUMMARY
The Problem. Although it is believed that there are many reasons for being able to achieve superior high temperature processing of materials in microgravity, major commercial applications are still being developed. A major cause for this is that the removal of gravity as a driving force has led to the discovery of a series of more subtle phenomena such as surface tension that have limited successful materials processing in space. These phenomena have so far been inferred and investigated based on the final state of materials that have been processed in space. Most high temperature materials processing conditions are set by general principles, experience and iteration. Non-intrusive, in-situ diagnostics are needed for research and optimum process control in the presence of these effects. Furthermore real-time operator interaction through direct visual cues is crucial to the control of many experiments, allowing rapid iteration for the fastest possible results in a limited time. Full optical access to furnace applications is needed for diagnosis and feedback control of the subtleties of high temperature processes under microgravity conditions.
Small furnace windows are currently used to provide access for assessing crystal quality, but only small portions of the object can be viewed and these windows themselves cause heat disturbances that affect the growth process. Also, many semi-transparent materials of interest have a large index of refraction that makes single direction viewing of an entire piece very difficult. Transparent furnaces are in use, but at relatively low temperatures because these furnace systems cannot survive the temperatures and heat loads required at high temperature. Specifically, current reflective coatings designed to contain thermal radiation have severe limitations.
The Innovation. The innovation of this program is the use of advanced techniques for heat containment and high temperature optical materials to build a transparent furnace rated at 1200°C or higher. Advanced high temperature radiation containment methods, elimination of convection heat transfer, high temperature transparent materials, and improved transparent heater component design have been combined to achieve a significantly higher temperature rating compared with current technology. An internal gold coating and a vacuum jacket between the process chamber and the heat radiation containing outer mirror tube has resulted in a dramatic improvement in overall heat containment and power reduction. Furthermore, reducing the heat loads on the mirror has allowed conventional mirror coatings to be compatible with high furnace core temperatures. A high temperature transparent furnace will create many new opportunities for high temperature material processing research and process control applications.
BACKGROUND
Transparent Furnaces. Transparent furnaces have been used in ground-based research for many years, in a concept first invented and patented at MIT [1] and further developed by private industry [2-4]. Transparent furnaces are used for research in areas that include crystal growth, metal bonding, combustion, solar energy, biomass energy conversion, and others. The ability to observe processes within the furnace can provide information unobtainable from post-cooldown sample characterization. In the area of crystal growth the in-situ, non-intrusive observational capability of a transparent furnace leads to the ability to change the growth ampoule temperature and/or temperature profile. As a specific example, control of the visible onset of nucleation and growth of crystals is far superior to preprogrammed techniques that are typically used with standard furnaces.
A standard earth-based transparent furnace uses a thin gold mirror coated onto the wall of a quartz tube to surround the hot zone of a furnace and contain infrared (IR) radiation. A typical furnace being used for crystal growth is shown schematically in Fig. 1. The mirror coating has the characteristic that it is reflective for longer wavelengths of radiation in the IR and transparent at shorter wavelengths in the visible. Depending on the details of the coating, approximately 95% of the incident infrared energy is reflected, while roughly 80% of the visible radiation is transmitted. Typically over 90% of the total energy incident from an 850°C blackbody is reflected.
The thin film gold coating is an effective radiant heat insulator while simultaneously allowing a clear view of processes occurring within. Furnace heating energy is provided by one or more helically wound resistance heating elements that have a coil spacing large enough to permit good visibility through the coils. A quartz shield tube is located between the heater and mirror to prevent the outgassing heater material from coating the mirror, reducing its reflectivity. A quartz process chamber is mounted between the heater and the growth ampoule, acting as an impurity barrier for the growth ampoule and reducing hot spots. The crystal growth ampoule is located along the axis of the furnace inside the muffle tube.
The primary constraints on future transparent furnace development are in the areas of transparent shell materials and radiation containment coatings. Current technology uses fused silica tubes, whereas much higher temperature versions will use materials such as sapphire. Subsidiary issues are the binding of the reflective coating to the substrate and the ability of these coatings to survive high temperatures. Previously, multizone transparent furnaces have been limited to temperature ratings of approximately 1000°C.
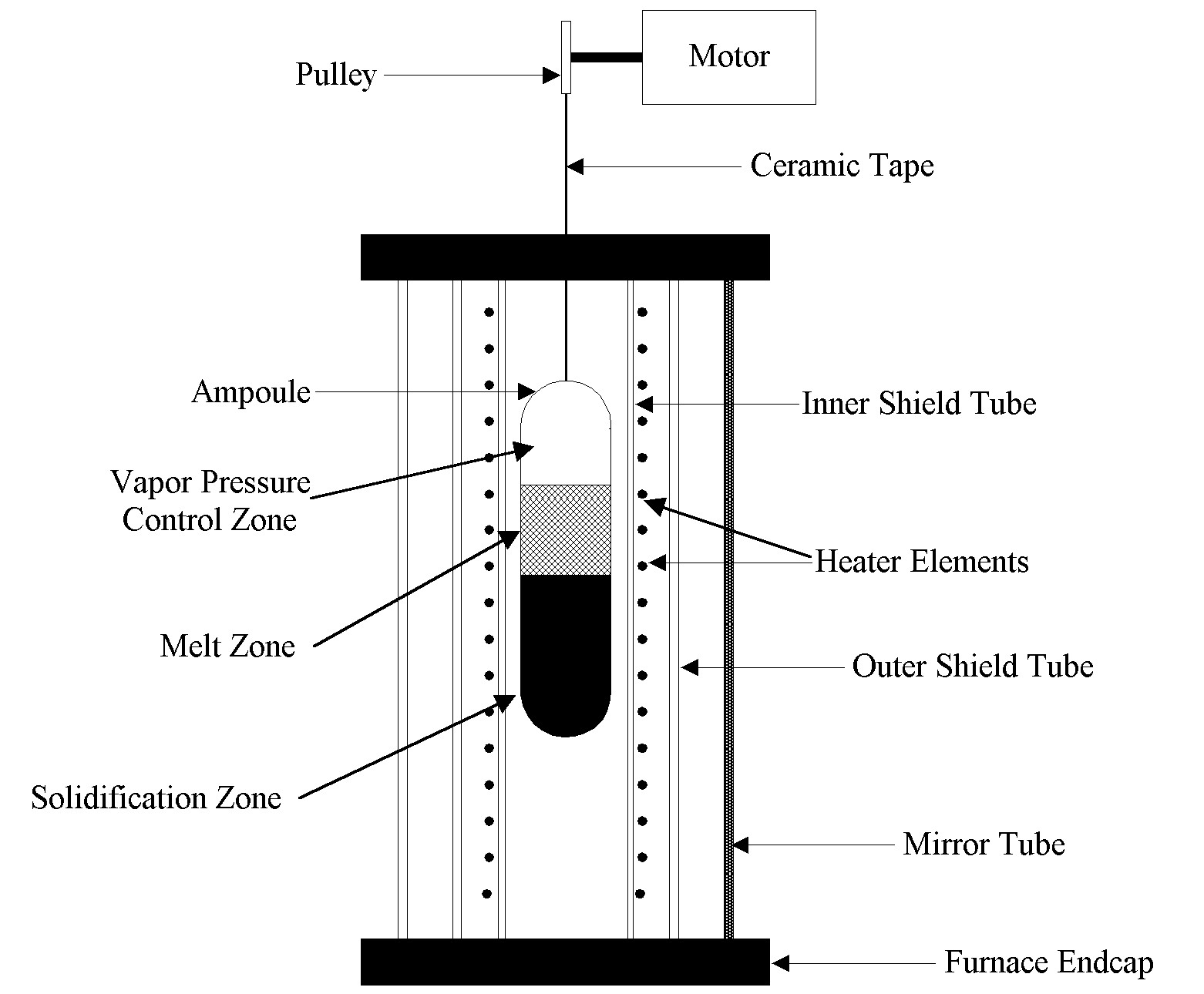
Figure 1. Schematic of standard Transparent Furnace used for crystal growth.
High Temperature Heat Transfer
At high temperatures radiant heat transfer is the dominant mechanism for heat loss, assuming no flowing or boiling liquids, and no large-area, high thermal conductivity solid contact between the hot zone and the environment. Radiation heat loss is defined by the Planck function as it describes blackbody radiation - a body that absorbs all incoming radiation.
W(l,T) = cλ / [(λ 5)(ec/λ T - 1)]
Where W(λ ,T) is defined as the power radiated per unit wavelength interval at wavelength λ by unit area of a blackbody at temperature T in measured in degrees K. cλ and c are constants. The total radiated power by a blackbody at temperature, T, is given by the familiar Stefan-Boltzmann function:
Wtotal(T) = 5.679 x 10-12 T4 W/cm2
The T4 dependence of the radiated power accounts for the dominance of radiation at high temperatures. For real materials the total radiated power differs from that of a blackbody by the total emittance, et, such that
Wreal(T) = et(T)Wblackbody(T)
Total emittances are integrals over wavelength of detailed emissivities that are also wavelength dependent. Total emittances can be quiet low, but also can depend strongly on temperature, usually increasing with temperature. Examples of commonly used materials are shown in Fig. 2.
Heat transfer as a result of convection and conduction can be estimated by using a thermal resistance concept:
Rthermal = Δ T/q
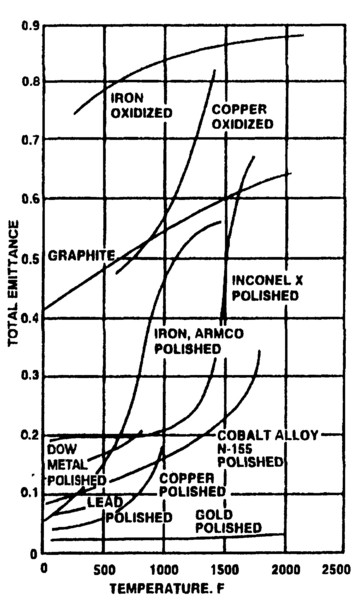
Figure 2. Total emittances of common materials versus temperature.
where ΔT is the temperature difference across the resistance path and q is the heat flux entering the resistance. A contact coefficient of heat transfer, hs, is R/A, where A is the contact area. For a wall, h = k/t, where k is the thermal conductivity, and t is the wall thickness. Orders of magnitude for h in W/m2-K are: 1) Gases in natural convection: 5-29, 2) Flowing gases: 11-290, and flowing liquids (non-metallic): 170-5700.
Convection can be eliminated by using vacuum enclosures. Conduction can be reduced by limiting contact area and using materials of low thermal conductivity (quartz). Thermal radiation can be reduced by using materials of low thermal emissivity.
Transparent Furnace Applications
There are many possible uses for transparent furnaces, including sintering, metal joining, and annealing, but current applications center on crystal growth. For this application, some of the capabilities introduced by a transparent furnace are: 1) Nucleation can be observed; if multiple nucleation sites occur solidification can be restarted, 2) The melt/solid interface can be viewed as a result of differences in density and emissivity between the liquid and solid, 3) Surface tension effects can be studied as a result of these liquid-solid differences. (This capability is important for microgravity research), 4) Convection can be studied through index of refraction changes with temperature, 5) Internal temperatures can be monitored by tomographic means, and 6) A variety of crystal defects are visible, depending on the optical properties of the crystal. Together, these advantages provide a powerful incentive to use transparent furnaces. The incentives will be further enhanced when standard optical diagnostics are produced to monitor processes in these furnaces. A case study in the actual benefits of transparent furnace use is given by Schunemann and Pollak [5]. In this work simple visual access to the process allowed drastic reductions (a factor of 10) in the time needed to develop a process for ZnGePh. An increasing amount of work is being done in the area of intelligent processing of materials [6,7]; optical access and optical diagnostics are playing a key role in this effort.
Crystal Growth. There are a number of techniques that can be used to grow crystals, including vapor phase growth [8], solution growth [9] and melt growth [10], are used to obtain crystals. Multiple zone electric furnaces with adjustable temperature profiles are usually used Bridgman crystal growth with vapor control of composition [11]. In a transparent furnace the quartz ampoule is the container for the material. The typical temperature profile has an isothermal in the upper zone, a 20°C temperature elevation in a middle region of 2.5 cm long at the melt-solid interface, and a bottom zone-cooling gradient of 25°C/cm. Crystals can be grown from a seed or seedless. The ampoule is translated through the temperature field and the crystal grows at a rate of 2-4 mm/hr.
Research has indicated that the achievement of improved crystalline and chemical perfection in single crystals grown using the vertical Bridgman technique is to a significant extent dependent on the ability to control the morphology of the growth interface [12], which will be visible using a transparent furnace. Recent analyses furthermore indicate that thermal conditions inherent to the Bridgman method, referred to as the "interface effect", in principle reduce the effectiveness of conventional means for controlling the interface shape.
The interface effect is due to a difference in the thermal conductivities of the melt and the crystal and/or the release of latent heat at the growth interface in the presence of a charge-confining crucible. Such conditions lead to a change in the axial temperature gradient of the charge at the growth interface whereas the axial temperature gradient of the crucible is continuous [12]. A temperature difference (and, thus, heat exchange) between the charge and crucible results, causing the interface shape to be nonplanar. For semiconductors, whose thermal conductivity of the melt is greater than that of the solid, the charge is cooled by the crucible, and therefore the interface shape is such that the crystal is concave. Experimental evidence for this behavior is provided, for example, by Lehoczky and Szofran [13] who first described the interface effect in order to explain observed segregation data in Bridgman-grown Hg1-xCdxTe. In the case of semi-transparent crystals this interface can be monitored using a variety of optical diagnostics, depending on the properties that change from the liquid to solid state.
TECHNICAL RESULTS
Transparent Furnace Heat Transfer Modeling and Analysis - Modeling of the heat transfer in the furnace used a MATHCADTM program to estimate heat transfer, developed into a version that allows an automatic iterative solution to the problem.
The modeling effort was first directed at the standard transparent furnace geometry and initial furnace modifications used achieve 1200°C operation. Next the model was converted to include a vacuum jacket and then concentrated on the radial radiant heat loads and end heat loads. Modeling switches were added to simulate the addition of various forms of end insulation to determine the impact of various schemes to reduce the end losses.
The model was examined to determine how well it predicted several phenomena previously explored. The first phenomena was the use of gas flow in an annulus inside the mirror tube to keep it marginally cool enough to survive high temperature operation in the furnace core. The second phenomenon was the lack of effectiveness of increasing the number of insulating shells; configurations with up to 7 shells were tested. The final results of Phase 1 showed rapidly diminishing returns with increasing number of shells, and this behavior was confirmed by modeling. The increase in insulation was found to be offset by the increase in radiating area as the tubes got larger.
Energy balance components were defined and ranked, and their variation with furnace size, shape, etc., was explored in order to optimize the prototype design. Specific objectives were:
1) Chart the contributions to the total heat transfer by conduction, convection, radiation vs. temperature.
2) Determine radial temperature profiles and coil temperature.
3) Determine the functional dependence on furnace parameters of Conduction, Convection, as well as short and long wavelength radiation.
4) Determine the effect on system temperature of large object inside - radiation.
The heat losses can be calculated from the thermal gradients predicted by the model. In the 1000 watt case for a non-radiative model the following are predicted:
.....................Loss..............................Power(watts)
......................Ends........................................25
Radial convective and conductive............854
.........Radiant(net)........................................135
________________________________
..............Total............................................1014...(1.4% error compared with 1000 w nominal power)
The next effort of the modeling and experimental testing was directed at higher temperature operation, emphasizing in the model the radiation behavior of the furnace. The outer mirror shell was added and temperature profiles compared for all core temperatures in this configuration. Finally the model was converted to have a vacuum jacket where radial radiant heat loads and the end heat loads control the heat transfer. Modeling switches were added to simulate the addition of various forms of end insulation to determine the impact of various schemes to reduce the end losses. The goal was to predict the split of power losses at higher temperatures, together with the effect on the system temperature of large object inside.
A primary purpose of modeling and experiment was to be able to predict radial and end power losses in the furnace resulting from convection, radiation, and conduction. Figure 3 shows power loss data for a 2-tube transparent furnace without a mirror tube at 5 different power levels (150 - 1300 watt). Actual total power levels varied from the nominal since the heater coil resistance was a function of temperature and only the on-time of the SCR heater control was used to set input power to the furnaces during experiments. The Case A bars of the graph and the Case B bars are for the 2-tube and 3-tube unmirrored furnaces respectively. The graph shows the reduction in convective losses and the increase in radiation losses when changing from a 2-tube to a 3-tube furnace configuration. Presumably this results from decreased convective flows as a result of a decrease in the temperature difference that drives the flows. Note that for similar input power levels the 3-tube unmirrored configurations have higher coil and core temperatures.
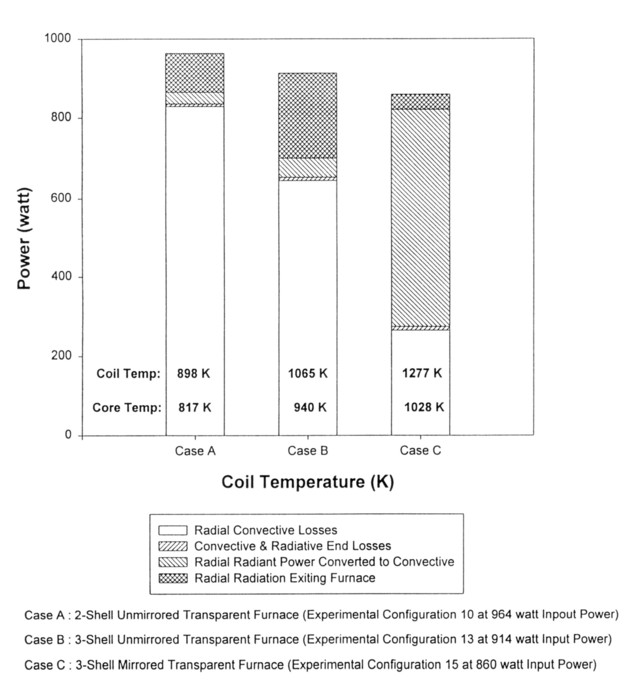
Figure 3. Energy Distributions for 3 Different Furnace Configurations at Nominal 1000 Watt Power Level.
Figure 3 shows three different furnace configurations at the nominal 1000 Watt input power level. This figure shows the changes in energy distributions as an unmirrored tube was added to the furnace, and then a mirror coating was added to the outside of this tube (left to right in the bar graph). Again note the significant increases in coil and core temperatures as one progresses toward a mirrored furnace for similar input power levels. End loss remain similar for all cases. More than 50% of the energy balance for the mirrored furnace is trapped radiative energy. This trapped radial radiant power is absorbed by the tubes and coil, increasing the furnace temperature. Higher temperatures are easily achieved using the same amount of input power and a mirror tube.
Figure 4 shows the temperature profile for the furnace as predicted by the model overlaid with collected experimental data for a nominal 626 Watt input power test performed on a 3-tube mirrored transparent furnace. The ratio of integrated areas for the modeled and experimental data gives a 1.2% error.
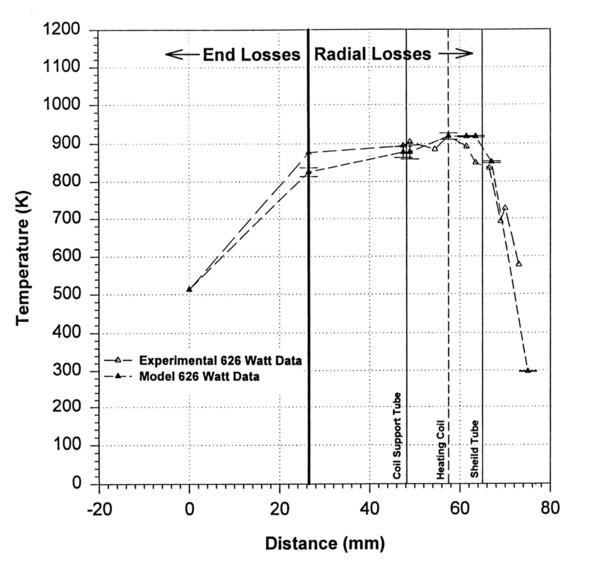
Figure 4. Comparison of Model and Experimental Thermal Profiles.
Further heat transfer modeling was done to verify the belief that convective heat transfer had not been removed at the vacuum levels achieved in the initial tests. Radiation heat losses were also estimated to determine how good the radiation confinement would have to be to reach furnace power levels well below 1 kW. The modeling was next used to guide alpha prototype experiments. There were three areas in which the modeling was involved; pressure threshold testing, radiation heat loss, and furnace power consumption.
For determining the pressure threshold for operation without convection heating convection, heat loss was monitored experimentally by a change in coil temperature associated with decreased heat losses. Modeling was used to give an indication of the relative fraction of total heat loss that was due to convection and radiation. Once convective heat losses were removed the furnace power would be predicted by radiation loss from the coil modified by any radiation reabsorption arising from radiation containment. This power would also then be directly monitored by the electrical power used by the furnace. In an unmirrored furnace where there is little radiation containment the furnace power should be proportional to Tc4, where Tc is the coil temperature. This was found to be the case in the experiments. Furthermore, the reduction in power as a result of radiation containment was be directly measured experimentally by a reduction in furnace power at the same coil temperature. The weakness of the alpha prototype was the lack of a mirror tube with an internal gold coating. An aluminum duct outside of an uncoated quartz tube was used to simulate the mirror tube, but its emissivity was too high and it absorbed too much radiant heat, which was then carried away by convection since it was outside the vacuum furnace. All of this behavior was found to be consistent with modeling.
Experimental results seem to confirm the importance of both vacuum insulation and the internal gold coating that prevents radiation absorption in the quartz. Very low power operation has been obtained.
Transparent Furnace Power Reduction - The primary technique that was used in this program to reduce furnace power was to create a vacuum jacket between the mirror tube and the outer shield tube. The primary purpose of the vacuum jacket is not to reduce power use, but to lower the heat flux and temperature of the mirror tube so that it can survive high temperature operation. The reduction in convective heat flux also reduces total heat power losses and thus reduces the electric power needed to reach a given operating temperature, since essentially all of the furnace electrical power is converted to heat. If the heat flow (including radiant heat flow) were totally contained the furnace would only use power to raise its own temperature. In the real device that uses a vacuum jacket the dominant channels of heat loss will be the radial radiant heat loss and end heat losses. Aside from work on the vacuum jacket this task will concentrate on these last two heat loss channels.
There are two mechanisms for reducing radial radiant heat losses. The first is to place the gold coating on the inside of the mirror tube without a protective quartz overlayer (previously the mirror coating was on the outside to make the coating deposition easier). Where the gold coating is on the inner surface of the mirror tube there is no direct absorption in the binder that keeps the gold on the quartz; there is only absorption in the binder after most of the radiation has been reflected by the gold coating. This approach was taken for the prototypes; the only difficulty being to find a vendor that coat the inside of the tube. The technique of using a simple line-of-sight evaporative coating from a boat can not be used on the inside of the tube as it was for the external coating. High quality internal coatings have been done in the past, but there are few commercial applications, so the process is not offered commercially.
The other major subtask was to reduce end heat losses. Radiant heat losses can be reduced be adding a reflector such as platinum or molybdenum foil, since no transparency is required for the ends. Insulators reduce convective and conductive heat transfer
Convection Heat Elimination. Preliminary experiments with the alpha prototype furnace showed large power drains on the furnace. Searching for the cause of this power loss, the amount that convection was reduced by the vacuum achieved was called into question. Research indicated that instead of being proportional to the gas density, the convective heating was proportional to the square root of the density through a dependence on the 1/4 power on the Grashof number for free convection heat transfer. Furthermore, gas conductivity decreases even more slowly with pressure down to 10 torr or less, then drops rapidly. These factors implied that to cut the convective heat losses by a factor of 10 would require at least a factor of 100 decrease in pressure. Experiments at different pressure were done in the alpha prototype confirming this behavior. Theory does not indicate the level of vacuum necessary to remove convection in our configuration; this was determined experimentally, these and these experiments defined a maximum allowable operating pressure for the furnace. The final prototype was easily modified to satisfy the new vacuum requirement by increasing the size of the pumping port.
Internal Gold Mirror Coating. Placing the gold coating on the inside of the mirror tube greatly reduces the radiation absorbed in the mirror tube and consequently its equilibrium temperature. The normal mirror coating on the outside allows absorption both in the quartz and in the binder layer for the gold before it is reflected from the gold. Placing the gold coating on the inside without a protective overcoating (not needed for an internal coating) eliminates both of these effects. The problem is that applying a uniform thin film to the inside of a tube is a difficult procedure of limited commercial application.
Internal gold coatings are usually chemically plated on or coated by evaporation coated from a central source of gold. One relatively easy method of evaporation coating of gold on the inside of a tube is first to electrochemically plate gold on a tungsten wire and then heat the wire electrically to evaporate the gold onto the inside of the quartz tube. A chromium binding layer is deposited in a similar manner.
A vacuum chamber at Thoughtventions was adapted for the gold evaporation process onto the quartz mirror tube. The initial apparatus consisted of a mount for the evaporation wire, high current feedthrus, a rotating drive mount for the tube, and inspection ports to monitor the coating process. The tube to be coated was set on vacuum bearings and rotated around the wire axis by a motor inside the vacuum chamber. Rotating the tube averages out any non-uniformities in the deposition pattern and leads to a much more uniform coating thickness on the quartz tube.
Gold coatings with and without a chromium binder were tried. The plated wire was heated to approximately 1000°C to evaporate the gold and deposits it on the tube. A HeNe laser aimed at the surface of the tube while it rotates was planned to measure the real time coating reflectivity to determine the correct length of time necessary for coating. The initial coating goal was a coating thick enough to transmit approximately 20% of the laser radiation. The reliability of the apparatus was to be tested during these experiments
Chromium deposition was attempted first because the chromium coated tungsten wire was inexpensive and easy to obtain. The chromium-plated wire was fixed into mounts in the vacuum deposition chamber and the wire was heated to an estimated temperature of 1200°C. A thermocouple was in contact with the wire to measure its temperature but was dislodged during the heating process. The chamber vacuum was maintained at 400 millitorr during this test, and at this pressure the surface of the chromium coated tungsten wire was found to oxidize, creating Cr2O3 and changing in color from silver gray to black. No deposition was detected on the quartz, but except for the high pressure the apparatus appeared to function properly.
A gold-coated tungsten wire was next used to attempt gold evaporation deposition on a cleaned tube. The vacuum was again about 400 millitorr and the wire temperature was estimated to be about 800°C. Evaporation of the gold resulted in the formation of in a golden black coating on the inside of the tube. The coating was only loosely deposited on the quartz tube, rubbing off easily. The golden black color would be consistent with a gold film deposited on an uncleaned substrate at relatively poor vacuum. During this experiment the rotating drive for the quartz tube failed when the rubber friction coupling between the motor and the tube melted; towards the end of the deposition, the tube was not turning.
Calculation of evaporation rate based on wire temperature and vapor pressure leads to an estimate of the deposition time. The rate of evaporation W of a metal is given by the equation:
log (W) = A - B/T - [log (T)]/2 + C
where W is the rate of evaporation expressed in g/sec-cm2, T is the absolute temperature (K), and A, B, C are well known constants. For gold, assuming a temperature of 1000°C gives a vapor pressure of 10-4 torr and an evaporation rate on the order of 10-6 to 10-7 g/sec-cm2. For chromium, 1200°C was assumed, giving a similar vapor pressure and the evaporation rate. The deposition time for a film of specific thickness is then estimated from the evaporation rate, which will guide the deposition process.
Based on the initial testing results a new fixture shown in Figure 5, for the evaporation deposition of internal gold film was designed and built, to improve the quality of the deposited film. Two pieces of tungsten wires were be mounted in the chamber to permit deposition of the chromium binder followed by the gold film overcoat without breaking vacuum, eliminating the possibility of oxidization of the chromium binder layer before the gold is added. Chromium is easily oxidized even at relatively low temperature, especially in the form of a very thin film (a few tens of angstroms) and would lose its ability to act as a binder.
The final apparatus consisted of a mount for the evaporation wire, high current feedthrus, a modified rotating drive mount for the tube, and inspection ports to monitor the coating process. The tube to be coated rests on vacuum bearings and is driven around the wire axis by a motor inside the vacuum chamber and connected to the quartz tube using a chain drive around a sprocket and graphite felt around the tube. The tungsten wires are clamped in 12 mm diameter water cooled copper rods that are powered through vacuum feedthrus. The entire apparatus except the motor is designed to be bakeable. Experiments were done to determine if the quartz tube could be baked out using the wires alone below evaporation temperature, but this was found to be impractical. The addition off long copier lamps solved this problem, heating the quartz tube to well over 200°C.
As before first a thin layer of chromium was evaporated onto a clean, baked out quartz tube. Chromium was deposited until a faint blackening of the tube was observable. The tube was then removed and inspected. The chromium layer was found to be uniformly deposited and so strongly adherent to the quartz that it could not be cleaned off. The evaporation was done at a 10-6 vacuum after the entire apparatus was baked out; the outer steel shell was baked over 100°C, and the quartz tube was baked out at over 200°C. The rotating mechanism worked well as did the water-cooled wire holders.
The next attempt included both chromium and gold. The chromium was evaporated as before, and then one of the gold plated wires was used to add a layer of gold. The gold coating was deceptive in again appearing visually black. Since the chromium also was black, it was difficult to detect whether a gold coat had been deposited, but it could be determined that all of the gold had been evaporated from the wire. After cooling down the apparatus, it was found that a coating had been deposited, but was very thin. Since the coating presumably was enough to protect the chromium, the last of the gold plated wires was inserted, and more gold added. This coating was also too thin, demonstrating that the plating on the wire was too thin for the required coating. Gold foil was then ordered and wrapped around a fresh tungsten wire. The wire was then heated and more gold deposited. A sufficiently thick coating was deposited, and the tube removed for use in the furnace. HeNe laser measurement indicated that the transmission was 0.1% at HeNe wavelength at the center of the tube. The ends of the tube were not coated at all, but coating here was unnecessary, since the radiation shields well in from the end of the furnace effectively end the radiation zone of the furnace.
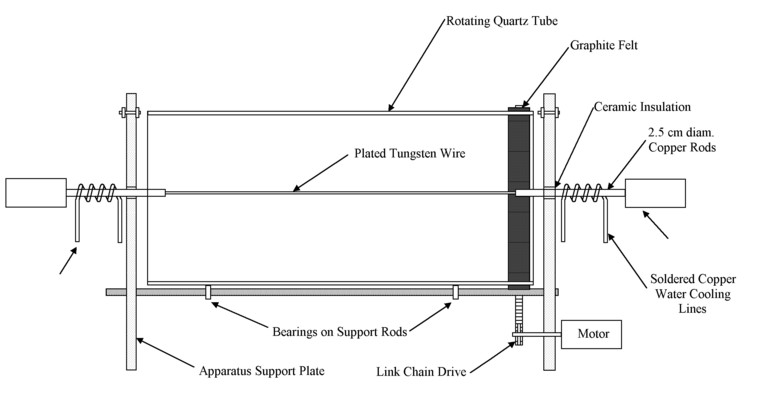
Figure 5. Gold coating apparatus schematic.
End Losses End heat losses consist of radiation escaping from the furnace ends, axial conduction along the tubes, and gas convection away from the core down the center of the muffle tube (assuming a vacuum jacket). The standard means of reducing each effect through the addition of insulation layers will be incorporated into the design. A potential problem was the requirement that the endcaps be cool enough to allow the O-rings to survive. This leads to a larger temperature difference and larger heat transfer at the furnace end, both as a result of convection and conduction. Prototype tests showed that there is no problem keeping the aluminum end flanges (and the O-rings in them) at low temperature using minimal water cooling.
Radiant. Radiant heat losses can be reduced by adding a series of radiation shields that have low emissivity. Each shield reflects much more radiation than it absorbs, and reradiates what it absorbs. Since the incoming radiation on the side facing the furnace core is a factor of 5 (emissivity = 0.2) more than that radiating from the other side, a series (4 or 5) of such shields allows a significant temperature drop between each shield, and a large total temperature drop across the entire shield package. The total radiation loss is not large. C/VI has a great deal of experience with such shield packs and has defined the basic design for this system. The rough rule of thumb is approximately 100°C temperature drop per shield. In the present case the shield pack is designed to decrease the temperature from 1200°C at the furnace core to approximately 800°C at the outside of the shield pack, which is a temperature at which radiant heat transfer is small and conventional ceramic cloth packing becomes an effective insulator.
Molybdenum shield packs were made. One molybdenum shield pack was cut and wired together for the prototype. Another was made at C/VI for inclusion into the final prototype according to the latest design information. One concern about the shield design is the support structure, which may need to support considerable (relatively) weight of the internal components of the furnace. Another concern is the possibility of corrosion/oxidation of the molybdenum, which has already appeared to some extent during preliminary testing of the alpha prototype. This presumably will not be a problem if the proper vacuum is maintained between the process chamber and the mirror tube.
Convection. Convection away from the core can only be reduced by inserting a thermal insulator at each end (with added radiation shields). This insulation would be in the form of blocks mounted on the workpiece support.
Conduction. The amount of heat conduction down the various tubes in the furnace will be determined by the thermal resistance at the end of each tube. It is planned to terminate some tubes before they reach the end of the furnaces, supporting them with rods through which heat conduction would be determined by the material used (quartz or alumina). The primary axial tube conductive loss will be through the muffle (central) tube because of the higher thermal conductivity of sapphire and alumina relative to quartz. The heat loss in the muffle tube will be determined by the thermal gradient along the alumina that will be established to permit the use of stainless steel at the ends rather than more expensive higher temperature materials such as inconel.
TRANSPARENT FURNACED DEVELOPMENT AND EXPERIMENTS
Vacuum Jacket. This was the most important subtask of the entire program. The key element of the high temperature furnace is the vacuum jacket. This vacuum jacket protects the mirror tube from degradation at high temperature by reducing the heat load on it. The jacket also reduces the power consumption of the furnace by dramatically reducing the radial heat loss. The heat loss is reduced by totally removing convective heat transfer and by improving the heat rejection efficiency of the mirror coating. It was estimated that a power reduction of a factor of 4 from that measured during experiments; without vacuum, insulation 800 W vs. 3200 W.
The overall design of the furnace became simpler as the project progressed. The first major design change occurred when the location of the high temperature sapphire vacuum wall (designed to survive pressurized operation at 1200°C) was changed from being the outer shield tube (next inside the mirror tube) to being the process chamber (the innermost tube). Originally it was thought best to make the vacuum jacket one integral unit and use radially adjacent tubes. However, using quartz as the process chamber might severely limit the usefulness of the furnace because quartz will devitrify readily at 1100°C as a result of exposure to many types of vapor. Such vapors will often be emitted by a sample being treated in the furnace. Sapphire is resistant to almost all chemical reactions, even at high temperatures. It was also realized that vacuum sealing at the ends must be done using demountable flanges, so that the same fabrication and assembly problems are encountered no matter which tube is made out of sapphire. One incidental benefit of using sapphire for the process chamber is that the sapphire for the tubes is much cheaper as a result of the significantly smaller diameter. Unfortunately sapphire tubes of the required length for the furnace are very expensive; the final tube must be made from shorter tubes.
The vacuum jacket was divided into 3 basic components, the process chamber, the end caps, and the mirror tube, and a whole series of parts within these three components: The process chamber was to consist of a sapphire center section and some transition structure to join the sapphire to the end caps. The end caps were to contain all of the demountable vacuum seals and vacuum feedthrus. The end caps also were to provide the physical and external mounting points for the entire furnace. The mirror tube was to be the outer furnace tube and radiation containment vessel to be sealed to the end caps, but not permanently. Primary design questions were the length of the mirror and process chamber tubes, the sealing techniques, and component layout.
Process Chamber Fabrication Development. Overall Context. The problem addressed in process chamber development was to create a tube with a transparent center that would both survive 1 atm. internal pressure and an external vacuum at 1200°C, as well as tolerate the axial thermal gradients from the 1200°C core temperature to room temperature at either end of the tube and the associated thermal cycling during furnace turn on and shut down. Quartz tubes would survive the axial gradients but are especially susceptible to devitrification at high temperature and have questionable strength at this temperature. Sapphire would easily survive the core temperature and pressure, but it is not commercially produced economically in long enough pieces of sufficient quality, does not tolerate thermal gradients or thermal shock well, and is very expensive. Extensive research was done to find a commercially practical solution to these material, thermal, and cost constraints.
The basic goal of the research was to create a process chamber permanent assembly where the center of the tube in the furnace core was sapphire, and the sapphire was joined to some other material at each end that would survive the thermal gradients. Finding a material to join to the sapphire was one problem that was addressed. The other problem was how to create the joint between this material and sapphire such that the joint could not only survive long term operation at 1200°C, but could survive the thermal cycling between 1200°C and room temperature.
The joint and end connection design was also important because the overall length of the process chamber assembly controlled the overall length of the furnace. The length of the process chamber was determined by the length of the sapphire (designed for full visibility along the full hot core length) plus the length of the transition pieces between the ends of the sapphire and the end caps. The length of the transition pieces had to be specified such that the thermal gradient that resulted from the heat flow from the core to the furnace end (controlled by the thermal conductivity of the material) was not too great for the material to support. Minimal gradients implied minimal heat loss and low thermal stress in the material, but high end cap temperatures. Larger temperature gradients led to lower end temperatures, higher thermal stress, and possible failure. Extensive thermal stress analysis was performed to determine what temperature difference or temperature gradient would be tolerable for different materials. Estimations were made, but the problem was not a standard case. Either the gradients or the net temperature difference may be important, since local gradients can lead to high local stress, whereas an overall temperature difference controls the net expansion difference between the hot and cold ends, and thus the maximum overall thermal (shear) stress. Another important factor was that in this particular geometry the shear stress is large (not tension or compression). It was concluded that much higher axial temperature gradients could be supported compared with almost any other geometry, but that experimental testing had to be done to determine specific performance.
A number of material possibilities were investigated. The first possible candidate for the end tubes was some type of metal, which could then be easily joined to the end caps. A metal C-ring might be used for the high temperature seal to the sapphire, but such a seal would be marginal, and difficult to fabricate and maintain. A better solution would be some permanent braze type joint between the sapphire and the metal. One such technique was already known, but is proprietary to Thoughtventions.
Alumina End Piece. The existence of a high temperature ceramic seal joint created some very attractive possibilities in terms of the fabrication of the process chamber. First the center sapphire tube could be made much more cheaply in sections rather than one long expensive piece. Second it made alumina a prime candidate as the material to be joined to the sapphire at high temperature. Alumina has good response to thermal gradients and since its properties are so closely related to sapphire, presumably it could easily be joined to the sapphire center tube the above mentioned high temperature sealing technique.
The large gradients that can be supported by the alumina tubes would greatly ease the furnace construction, although they might lead to larger end heat losses than might otherwise be the case. If the axial temperature of the ceramic part of the process chamber could be brought down to 400 or 500°C, stainless steel end pieces could be used, as well as standard welded bellows. Welded (rather than formed) bellows are much preferable as a result of the much-reduced overall length required to absorb the 4 mm thermal expansion of the process chamber tube. Extensive research indicated that a formed bellows that could absorb this movement and high temperatures would have to be approximately 20 cm long, whereas the welded bellows need only be 5 cm long, and important reduction for a device that may be greatly constrained by use on the space station.
The bellows configuration of the process chamber tube was then significantly simplified by the elimination of the bellows at one end. By adding radial O-rings in the end caps to seal the process chamber tube, this tube would not be fixed axially in the furnace and the assembly could accept axial thermal expansion of the tube naturally. This would make the entire muffle tube simpler and easier to fabricate. The current design then consisted of 3 central sapphire tubes with an alumina tube followed by a metal tube on each end. This design may be simplified further by eliminating the metal tubes as a result of a desire to make the muffle tube a separate piece in the assembly rather than making it integral with an external vacuum fitting. This separation would protect the primary high-cost piece of the furnace, the sapphire.
Experiments were then done to determine how large a thermal gradient alumina tubes could support. High purity alumina (Coors, 99.8% alumina) tubes were purchased for these tests. One end of the tube was placed in a 1200°C tube furnace, the packed around its outside and inside with insulation, and the other end was left in room air. When heating one end of the tube in this configuration did not damage the tube, cooling fins were attached to the tube and a 1200°C to room temperature transition was made in less than 5 cm, again without damaging the tube after multiple tests. Since this thermal gradient was a more severe test than could possibly be achieved in the furnace these tests confirmed that alumina could be used as the end transition pieces.
The design was thus further simplified to be made up of 3 sapphire tubes with alumina tubes at the end; eliminating the metal tubes and greatly easing the overall fabrication of the process chamber. The alumina tubes are adequately sealed by O-rings in the end flanges, and heat conduction down the alumina was not large. Process Chamber Final Options In the delivered version of the furnace a quartz tube was used as the process chamber. Investigation into the use of quartz in the electronics processing industry showed that it had been used successfully at temperatures over 1400°C. Use was under vacuum, but indications were that it had sufficient strength at 1200°C to support 1 atm. as a column pressurized from the inside, providing care was taken to prevent devitrification of the quartz (quartz is more sensitive to this process at higher temperatures). The quartz tends to sag under gravity after extended periods of operation, but this behavior can be compensated for by rotating the quartz tube periodically. All testing at 1200°C showed that the sagging takes place over periods significantly greater than 10 hours. The 100 hour test of Phase 1 showed no sagging. The quartz process chamber can simply be replaced, or a shield tube can be placed inside it to prevent contamination and devitrification.
The use of a quartz process chamber does limit the furnace to not much more than 1200°C operation, although the other quartz tubes, the mirror surface, the coil and the entire furnace are apparently capable of significantly higher temperature operation.
Heater Elements. Standard high temperature heater elements were used; a molybdenum heater element. The large change in resistivity makes the use of this material different than kanthal-based materials both in terms of diameter and power supply specification. The coil parameters (coil diameter, wire diameter, coil spacing, coil length were specified based on a calculation of the details of the resistance versus temperature of molybdenum. The coil spacing was halved at the end of the hot zone to improve core temperature uniformity over the full 20 cm hot length.
End Insulation. The end insulation consists of radiation shields and the vacuum insulation that removes convection heat transfer in the space between the process chamber and the mirror tube. The shields are made of polished molybdenum and a series of 5 shields were used. There is typically a 100°C temperature drop per shield, so to reduce the 1200°C core temperature to a non-radiative temperature requires about 5 shields. Fibrous ceramic completes the insulation package.
Mirror Tube/IR Mirror Coating. Some experiments were performed to find out why gold coatings on flat mirror survive at higher temperatures than apparently identical gold coatings on tubes. Speculation at the end of Phase 1 was that the difference between these cases was that one quartz surface was ground and polished (flat window) whereas the other was drawn and microscopically smooth. Some experiments were performed to discover the cause for the failure of the gold coating at 500°C.
Initial microscopic inspection of a failed coating implied that spalling was the failure mode, indicated by small crater-like marks in the surface. The coating usually failed over restricted areas, not simultaneously over the entire hot area, and it failed within these specific areas at widely distributed small spots. The greater the degree of failure of the coating, the higher the density of the spots, until catastrophic failure resulted in the loss of the coating over wide areas. SEM examination of a piece of a mirror tube with a failed coating provided a detailed picture of the spots. Normally, significant amount of cracks or sharp edges would be expected around the spots if the coating was spalling off, but the pits appeared rather to be burned or oxidized at high temperature. It seems that the temperature at some spots on this sample was quite high, since gold is not oxidized below 800°C. The mechanism for the oxidation might be that the quartz film had spalled off as a result of high thermal stress, leaving the underlying chromium and gold coating exposed to air and subsequent oxidation.
The failure of apparently random large areas implied a failure mechanism associated with a non-uniformity of the coating. The thin chromium bond layer may be too thin in some areas, causing insufficient adhesion of the gold coating, and leading to the spalling off of the entire Cr-Au-Cr-SiO2 coating sandwich. The cleanliness of the coating process may also have been a factor, where isolated impurities reduced the coating adhesion locally.
There are a number of natural temperature limits to a SiO2-Cr-Au coating. The diffusion of chromium into both gold and quartz, and the interdiffusion of all the different types of films was studied for the case of the mirror coating. Specific diffusion data for chromium or gold into quartz was not available, so direct estimation of the diffusion parameters could not be done. However, the diffusion coefficient of nickel into gold was found and the diffusion process was checked at temperatures between room temperature and 800°C. Below 500°C, the diffusion effect is not significant. Above 1000°C, thin layers diffuse into the bulk material quickly and lose their capacity to act as independent layers.
The diffusion processes are of three basic types: 1) metal diffuses into metal, 2) metal diffuses into ceramic, and 3) oxygen diffuses into ceramic and through ceramic into metal. Metal diffusion into ceramic is very slow compared with metal diffusion into metal because most ceramics act as ionic solids to some degree, and diffusion in ceramics is an ionic process, which is an inherently slower process than metal atom diffusion. Also the solubility of most metals in oxides is also low. The primary mechanism is thus the diffusion of metal to metal, and diffusion of oxygen into ceramic and metal. To be certain, the diffusion of metal into ceramic was calculated and this calculation confirmed its relative unimportance.
The primary concern in the specific case of the transparent furnace mirror coating is the diffusion of chromium into gold. About 5 wt% of chromium dissolution in gold will lead to the formation of the α ′ ′ phase of gold at 500° C according to the Au-Cr binary phase diagram. The presence of this new phase would lower the IR reflectivity of the gold layer or cause the entire layer to delaminate as a result of to the volume change of the coating during the phase transformation. The rate at which this phase is formed is believed to be diffusion controlled, since sufficient chromium concentration must be built up in the gold layer. The primary question is whether chromium has a diffusion rate high enough in gold at 500°C to allow the α ′ phase to form in a relevant time. The diffusion coefficient of chromium in gold was found from a paper recently by Rairden, et al. [17]. At 500°C, the diffusion coefficient is 10-16 cm2/sec. The time needed for chromium to diffuse into a 1 µ m thick gold layer at 500°C was estimated to be tens of thousand hours.
t = (2π)2/πD = (2 x 10 -4)2/(3.14 x 10-16) = 3.5 x 104 hours
This implies that the diffusion of chromium into gold at 500°C is not significant and would not cause a major adverse effect to the reflectivity or debinding of the coating.
Quartz is a strongly bound covalent network, the diffusion coefficients of the network formers (Si+4 and O-2) are very low, as are the diffusion coefficients of non-alkali and non-alkaline earth metal impurities in the quartz [18]. For example, the diffusion of nickel into quartz is slow: at 500°C, D=10-20 cm2/sec. The estimated time for nickel to diffuse into 1 µ m thick quartz layer at T = 500°C is about 3.5 x 108 hours. The diffusion coefficient of chromium in quartz has not yet been found, but it should be of the same order as nickel, so that the diffusion of chromium into quartz should be negligible.
Gaseous oxygen in air diffuses into oxide-based ceramics as an interstitial oxygen molecule. This oxygen diffuses rapidly but does not interact readily with the network oxygen in quartz [18]. The diffusion coefficient of oxygen in SiO2 is found to be 10-12.5 cm2/sec at T = 500°C. This calculation shows that at 500°C, the oxygen can diffuse through 1 µ m thick SiO2 layer in a few hours to reach the chromium bond layer.
There is a possibility that Cr2O3 would form if the oxygen reacts with the chromium binder. Cr2O3 is particularly optically absorptive; it is a material well known for its broadband, near-perfect absorption. The temperature near any Cr2O3 formed would be quite high, and might cause the failure of the gold layer. Cr2O3 formation as a result of oxygen-chromium reaction was estimated using thermodynamics calculations. The Gibbs Free Energy of Cr2O3 at 500°C was calculated and from that the oxygen partial pressure was derived. The calculation showed that at 500°C, Cr2O3 can be formed at an oxygen pressure of 10-38 atm. This indicates that chromium oxide can be formed at a very low oxygen pressure at 500°C.
This calculation was confirmed by previous experimental work [19]. They investigated an Au-Cr thin film system annealed in air between 280°C and 450°C; the reaction was studied by measuring resistivity changes, and performing SEM, and TEM inspection. In their work, thin gold films (8000Å and 500Å) were deposited on glass substrates with chromium as a bond layer (300Å) between the gold films and the glass substrates. A Cr2O3 layer was found over the entire exterior surface of gold after annealing in air. Diffusion of chromium through gold layer to reach the surface and react with oxygen was postulated as the mechanism for these results. The sequence of events appeared to be that the chromium diffused through the gold grain boundaries and lattice to the outer surface of the gold layer and then was oxidized there to form Cr2O3. The formation of Cr2O3 than became the driving force for more chromium to diffuse to the surface until the chromium layer was totally consumed. The chromium diffusion and Cr2O3 formation was observed on both surfaces of 8000Å and 500Å films; the process was faster for the thinner gold film.
Diffusion of chromium into SiO2 is much more difficult than the diffusion of chromium to gold, so that in the present case it is oxygen from the atmosphere that diffuses through the SiO2 layer to reach the chromium layer, and form Cr2O3 at the chromium/SiO2 interface.
The formation of Cr2O3 at temperatures above 300°C seems inevitable. This would explain the failure of the gold mirror coating reported in the Phase I final report: oxygen diffuses through SiO2 to reach SiO2/Cr intersection and reacts with chromium to form Cr2O3. Individual Cr2O3 particles form first between SiO2 and chromium layers and keep growing. These Cr2O3 particles absorb radiation, which causes a local temperature rise and results in the thermal spalling. This phenomenon would explain the small spots that were observed during the initial stages of the mirror coating failure it Phase 1 work. The Cr2O3 particles would grow with time and gradually form thin Cr2O3 layer (either continuous or discontinuous), leading to mirror coating failure in relatively large area as was observed. This argument might also explain the difference in survival temperature of flat plates vs. round tubes, simply because the flat pieces were tested in a furnace, where the radiant heat load is much lower. Apart from diffusion mechanisms, coating deposition quality is another important issue that controls the performance of IR mirror coating system. It is normally more difficult to control the deposition process for curved surfaces than for flat ones, especially during large area deposition. A number of depositions may be needed to coat the entire area, so that control of overlapping areas is difficult. The thickness and uniformity of the thin IR mirror coating are difficult to control during the deposition. The unevenness of each layer may result in poor adhesion or thermal stresses at temperatures around 500°C, which would eventually cause the degradation of the coating.
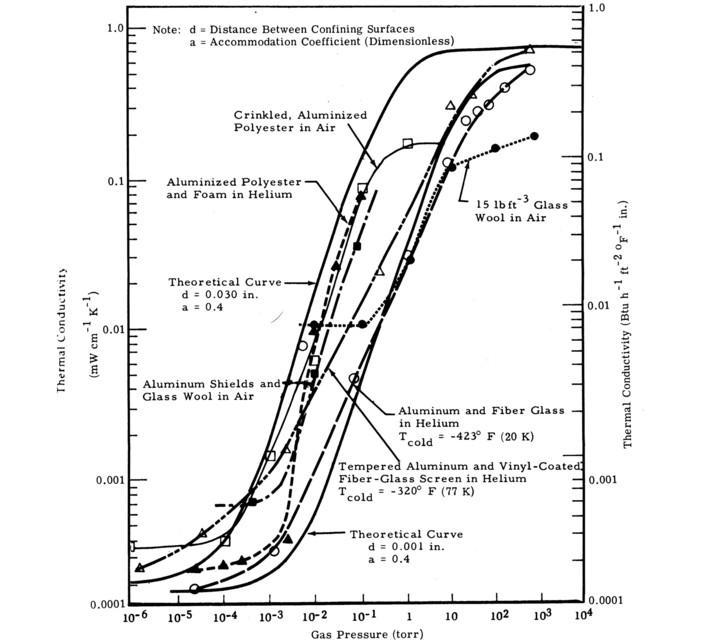
Figure 6. Effect of gas pressure on thermal conductivity [20].
4.3.4. Prototype Transparent Furnace Construction, Testing and Delivery. This task involved the construction of the final prototype to be delivered to NASA. The overall work fell into the categories of: 1) Transparent Furnace, 2) Vacuum, Electrical, and Control Subsystems, 3) Cabinetry and Mounting, and 4) Computer Control and Instrumentation.
Transparent Furnace. Shown in Figure 7 is an accurate side-view half-scale assembly cross section drawing of the prototype transparent furnace. The furnace is primarily an assembly of cylindrical components with the exception of the heater element support rods. Detailed assembly procedures were developed during prototype fabrication, with appropriate modifications to the design to ease the process. Putting together an enclosing multiple (fragile) shell device can be quite complicated.
The major problems that were encountered were: 1) being able to have access to the full process chamber tube bore for work insertion, and yet have thermocouple and heater element feedthrus at a slightly larger diameter, 2) designing one end so that all of the internal connections could be made after the end flange was put in place, and 3) assembling and connecting the insulated heater element. The final goal was that the furnace be assembled easily without extreme care, and that the design tolerances would assure that standard assembly procedures could be used for the most part. As a commercial product, simple and easy assembly may make the difference between a good or mediocre product.
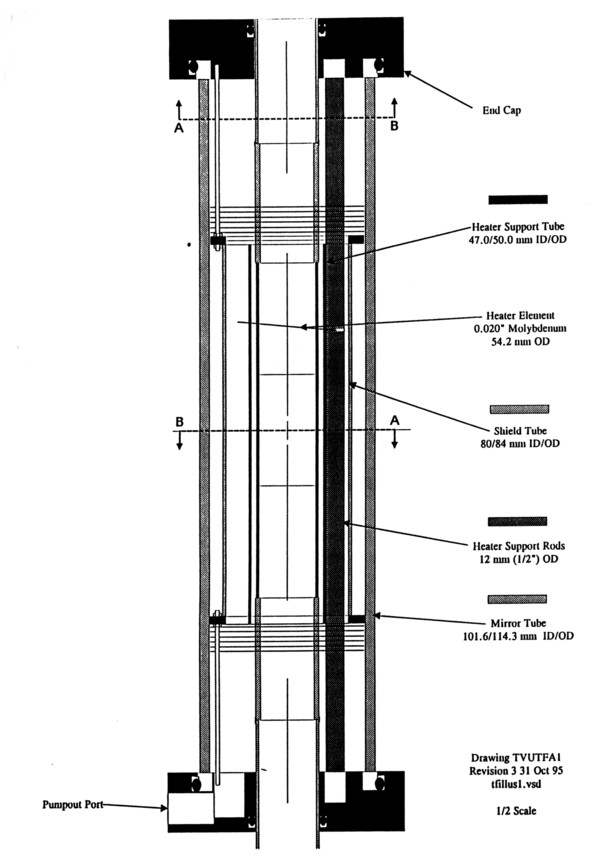
Figure 7. Prototype Transparent Furnace Cross Section Drawing.
Design changes made to improve the manufacturability of the furnace with respect to past models have been successful. Previously the coil supports used a complex quartz structure done by a skilled glass blower. The current model uses inexpensive independently slotted rods and simple quartz tubes. Also, the vacuum system uses only O-rings and KF fittings, so that the components are inexpensive and easy to replace, and the system is easy to assemble. These are all major advantages for final product, and should ease the final commercialization.
Initial attempts were made to wind a molybdenum coil and test it in the prototype assembly, and to evaluate the adequacy of the 40 Amp SCR power supply to be used to heat the coil. It was discovered that the molybdenum wire was much stiffer than the kanthal wire and has a lower spring factor. This made it impossible to prepare a coil on the slotted quartz support rods while they were in place as was done using a kanthal coil. The coil had to be wrapped on a preforming mandrel such that after the release of tension it was as close as possible to the final diameter of the slots in the mounted quartz rods. Otherwise the stress and torque on the quartz rods (already weakened by the slotting) will break them or severely chip the corners of the slots. The first attempts at winding the molybdenum around fixed position quartz rods broke several quartz rods (some of which could be re-fused) due to the remaining tensions in the coil. Additional slotted rods were ordered with reduced slot depths (changing from 6mm depth to 4mm) to improve the strength of the rods. Molybdenum has approximately half the thermal coefficient of expansion of kanthal so reduced slot depth should be sufficient to keep the molybdenum coil in place while strengthening the quartz rods.
The vacuum system was assembled and interfaced to the furnace end plates. The electrical and control systems were next assembled. The furnace coil was wound and the quartz shells prepared for final assembly. Problems encountered during assembly included vacuum leaks, winding the molybdenum coil on the slotted rods, and being able to make the final heater connections before adding the second end cap to the end of the furnace. To aid this process extension sleeves were made for the quartz rods so that they could be slid out for coil attachment and slid back as the end cap was put on.
Vacuum, Electrical, and Control Subsystems. A CVC 2" water cooled diffusion pump, water cooled baffle, and electropneumatic gate valve form the high vacuum system; an E2M2 Edwards direct drive roughing pump (2.0 cfm/min), inline oil/particle filter, and electropneumatic valves form the rough vacuum system. The high vacuum piping is primarily composed of nominal 1.5" diameter conflat fittings. The roughing system uses 1.0" quick flange type fittings and 3/4" diameter gum rubber hose.
The vacuum system was tested with the furnace in place. Only the furnace outer mirror tube was used for the vacuum testing. No internal coils, supports, end shields, or muffle tube were in place for this test. The system rough pumped to 50 mTorr when the largest o-ring seals were formed from nominal 4.5mm diameter o-ring cord stock. It is known, from experience with the prototype furnace system, to be difficult to make perfect o-ring joints by hand, so professionally assembled o-rings will be ordered to replace those made at Thoughtventions. The high vacuum diffusion pump evacuated the system through the 1-1/2 diameter muffle tube port to 1 mTorr as measured at the far end of the furnace by a Varian convector T/C gauge. The transparent furnace had a leak rate of 3.8 days to atmospheric. This leak rate should be easily improved with new o-rings and additional tightening of the T/C and electrical feedthrough fittings.
The final furnace will have less internal volume to be evacuated thereby improving the rough vacuum, but these elements will also disturb the flow patterns possibly reducing the ultimate achievable vacuum pressure. The final furnace coil system will also be pumped on initially through only 3/4 and 1" inch diameter fittings, which will reduce the speed with which the chamber can be pumped.
Computer Control and Instrumentation. The furnace is controlled by a PLC and an INTELLUTION software package (FIXMMI) that performs graphic systems control. This package is very flexible, but somewhat awkward to use. It is a higher level language, so it does avoid the FORTRAN code control that has been used with lower temperature models of the transparent furnace, which is difficult to modify. The package has the potential for rapid modification for varied future uses.
A user's manual was shipped with the furnace. The manual includes system specifications, assembly procedures, and operating procedures.
Final Testing. After assembly of the complete furnace system, extensive hardware and software system debugging was done to assure proper function of all components. Vacuum system manual and automatic control was verified, and all vacuum leaks eliminated. Sensing and heater power was tested. Safety interlocks were tested. Proper cooling was installed for all equipment, including the furnace end caps. Continual improvements were made in the systems and system fabrication techniques, approaching as closely as possible the commercial model of the furnace. An image of the final configuration is shown in Fig. 8.

Figure 8. Image of the final high transparent furnace system.
After verifying that the vacuum levels needed for elimination of convection were achieved, the furnace was powered. Initial tests were done using a clear quartz shell to visually verify that the response of the internal components of the furnace was appropriate. Low temperature power measurements confirmed that convection had indeed been eliminated at the vacuum levels achieved with the final vacuum configuration of the furnace. There had been some concern about this because pumping was only done from one end, and the central and far end of the furnace had to be pumped out through the radiation shields, which blocked the flow.
Finally the gold coated tube was added and further testing was done. Large power reductions were observed with the gold coated tube compared with a clear outer tube. The goal temperature of 1200°C was reached with a power of 1.4 kW. This is only 45% of the power supply capacity. The gold coating on the mirror tube was found to be too thick at the center and too thin at the outer edges of the hot zone. Some outgassing was observed during 1200°C operation, clouding the shield tubes.
CONCLUSIONS
A prototype 1200°C transparent furnace has been designed, developed, fabricated, tested, and delivered to NASA. Thermal modeling was developed and used to predict performance. High temperature operation at low electrical power is achieved by eliminating convective heat transfer and containing radiant heat. Convection heating is removed by creating a vacuum jacket between the process chamber and the outside shell of the furnace. Radiation containment is performed by using a infrared reflecting coating on the inside of the outside quartz tube that transmits enough visible light to view processes taking place in the furnace core. Radiation is prevented from escaping from the ends of the furnace by using metal radiation shields.
The furnace and its associated vacuum, electrical, and monitoring systems is computer controlled and packaged as commercial equipment.
Applications: There are many generic uses for transparent furnaces in materials processing and fluid and solid mechanics at elevated temperatures. Transparent furnaces can be used for research in crystal growth, sintering, metal joining, and annealing, high temperature materials properties, and the behavior of flowing systems that use high temperature liquids. In the area of crystal growth, some of the capabilities introduced by a transparent furnace are: 1) Nucleation can be observed; if multiple nucleation sites occur solidification can be restarted, 2) The melt/solid interface can be viewed as a result of differences in density and emissivity between the liquid and solid, 3) Surface tension effects can be studied as a result of these liquid-solid differences, 4) Convection can be studied through index of refraction changes with temperature, 5) Internal temperatures can be monitored by tomographic means, and 6) A variety of crystal defects are visible, depending on the optical properties of the crystal.
ACKNOWLEDGEMENTS
The author gratefully acknowledges the support of this work by the National Aeronautics and Space Administration under Contract No. NAS3-26665.
REFERENCES
1. T.B. Reed, "Transparent Furnace for Vapor Crystal Growth," Solid State Research Report, Lincoln Lab, MIT, 1, 21, (1969).
2. D.W. Yoel, and D.M. Garman, "Transparent furnace technology for space applications," International Astronautical Federation Congress, Paper No. IAF-87-047, October, (1987).
3. D.W. Yoel, and T.L. Thomas, "Telescience vapor crystal growth using small satellites," Proceedings of the 1st Annual USU Conference on Small Satellites, Utah State University, Logan, Utah, October (1987).
4. D.W. Yoel, and S.L. Sperry , Space-based processing of semiconductor materials by chemical vapor transport, American Astronautical Society Science & Technology Series, 67, AAS 86-559, H. Jacobs, Editor, (1987).
5. P.G. Schunemann, and T.M. Pollak, "ZnGeP2 Crystal Growth Studies using the Horizontal Gradient Freeze Technique," Presented at the Tenth Intnl. Conf. on Crystal Growth, Aug. 16-21, San Diego, CA, (1992),
6. P.H. Garrett, J.G. Jones, D.C. Moore, and J.C. Malas, "Emerging Methods for the Intelligent Processing of Materials," J. Matl. Eng. and Perf., 2, 5, 727-732, (1993).
7. N.M. Wereley, T.F. Zahrah, and F.H. Charron, "Intelligent Control of Consolidation and Solidification Processes," J. Matl. Eng. and Perf., 2, 5, 671-682, (1993).
8. C. Geibel, H. Maier, and R. Schmitt, Journal of Crystal Growth, 86, 386, (1988).
9. R. Triboulet, R. Legros, A. Heurtel, B. Sieber, G. Didier, and D. Imhoff, Journal of Crystal Growth, 72, 90, (l985).
10. K.Y. Lay, D. Nichols, S. McDevitt, B.E. Dean, and C.J. Johnson, Journal of Crystal Growth, 86, 118, (l988).
11. K. Mochizuki, and K. Masumoto, "Melt Growth of CdTe Single Crystals with Controlled Deviation from Stoichiometry," Mat. Lett., 6, 4, 119-122, Feb. (1988).
12. T. Jasinski and A.F. Witt, "On Control of the Crystal-Melt Interface Shape during Growth in a Vertical Bridgman Configuration for Crystal Growth," J. Crystal Growth, 71, 295-304, (1985).
13. S.L. Lehoczky and F.R. Szofran, Materials Processing in the Reduced Gravity Environment of Space, G.E. Rindone Ed., North-Holland, Amsterdam, 409, (1982).
14. S.C. Bates, "High Temperature Transparent Furnace Development," NASA Contractors Report, NASA CR 202333, April, (1997).
15. G.R. Van Houten, "A Survey of Ceramic-to-Metal Bonding," Ceramic Bull., 38, 6, 301-307, (1959).
16. W.E. Hauth III, "Ceramic-to-Ceramic Sealing of Large Shapes," Ceramic Bull., 58, 6, 584-6, (1979).
17. R.J. Rairden, C.A. Neugebauer, and R.A. Sigsbee, Metallurgical Transactions, 2, 719-722 (1971).
18. A. Atkinson, Reactive Phase Formation at Interfaces and Diffusion Processes, Ed. by Limoge Y. and Bocquet J. L., Materials Science Forum, Vols. 155-156, 245-256, (1994).
19. A. Munitz and Y. Komem, Thin Solid Films, 37, 171-179, 1976.
20. The Infrared Handbook,W.L. Wolfe, and G.J. Zeiss Eds., Infrared Information Analysis Center, Environmental Research Institute of Michigan, MI, p. 15-71, (1993).