Net Shape Bulk Diamond Fabrication
40 Nutmeg Lane
Glastonbury, CT 06033
ABSTRACT
Diamond is a material that has uniquely attractive physical, thermal, and optical properties. Small industrial diamonds have been grown artificially for a number of years, but chemical vapor deposition (CVD) techniques that have been used to grow diamond thin films have made diamond parts a reality for a wide variety of mechanical and optical uses. The high strength, extreme hardness, high resistance to thermal shock, and infrared transmissivity of diamond make it an ideal material, for many applications. A CVD diamond growth technique has been developed and patented that can be used to fabricate bulk diamond. Experiments have been designed and will be performed to demonstrate the growth of a diamond film using this method when funding becomes available. Film growth will be done to enhance analysis and description of the process.
INTRODUCTION
Overall Technical Objectives. The overall technical objective of the program is to develop and demonstrate a process closely related to diamond sintering. This will be done theoretically and experimentally defining both the proper vapor excitation conditions and the necessary substrate processing parameters. This proposed program is based on separate, previously published work that: 1) Demonstrates diamond growth by C2 addition in the absence of H2, and 2) Demonstrates photofragmentation generation of C2 fragments from C60 vapor.
Significance. Diamond is a material that has uniquely attractive physical, electrical, thermal, and optical properties. It is also a metastable crystal at room temperature that forms naturally only at extremely high pressures and temperatures. Small industrial diamonds have been grown artificially for many years and, more recently, various forms of chemical vapor deposition (CVD) techniques have become an established means used to grow diamond thin films that have a wide variety of mechanical and optical uses. All of these synthesis techniques practically produce diamond that must be thin in at least one dimension. In the case of industrial diamond, processing parameters limit practical sizes for diamond growth to a scale of a few µ m; CVD diamond films can be grown over large areas but the thickness growth rate is too slow to be practical for significantly 3-D structures. CVD growth processes also require special gas/plasmas conditions above the growing substrate that prevent the use of these processes in the small volumes that would be necessary to perform diamond joining.
The innovation of this work is the use of photofragmented fullerene diamond deposition and crystal growth to bond separate pieces of diamond into a monolithic diamond structure. This process can be used either to join separate pieces of diamond into a single structure, or to create an arbitrarily sized and shaped single, bulk net shape diamond part that has been formed from inexpensive, bonded diamond grit. The basis for this approach is separate work that shows that: 1) Fullerene, C60, photofragmentation occurs in response to excitation by a high intensity light source, and this fragmentation occurs in preference to ionization or other de-excitation processes as a result of the unusual nature of the resonant bonding in the fullerene molecule, 2) Diamond growth has already been demonstrated using fullerene fragmentation in an argon microwave plasma. Photofragmentation is a prerequisite for internal diamond deposition inside a small volume because the normal CVD process using plasmas is not possible on such a small scale. The proposed technique is effectively a means of sintering diamond that uses C60 vapor passing through pores in the diamond as the carbon source for deposition bonding, resulting in inherently porous diamond. Aside from creating large diamond structures, porous diamond has a variety of optical, heat transfer, and other applications.
BACKGROUND
Diamond. Diamond is an unusual material in many respects, with mechanical, electrical, thermal, and optical properties that surpass almost all its competitors (Table 1) [1] Specific properties of note are its hardness, strength, thermal conductivity, electrical resistance, electrical carrier mobility, and dielectric breakdown strength.
Diamond and graphite are the two most common crystalline forms of carbon. The exceptional properties of diamond are a consequence of the strength and symmetry of its atom-to-atom bonding structure. The electronic structure of carbon results in four sp3 covalent bonds to all of its nearest (and maximum possible) neighbors. Covalent bonds are the strongest type of electron sharing, and the carbon atom is small enough so that its electrons are also tightly bound. This combination makes diamond stronger, stiffer, and harder than any other known material. Compared with silicon carbide and sapphire (Table 1) diamond is approximately 4-6 times stronger, and 2-3 times as stiff (2-3 times higher Young's modulus). This unusually strong bonding structure is also responsible for the unusual electrical properties of diamond, with a large band gap and high resistivity.
Natural diamond is classified in four types. Type 1a (98% of natural diamond) contains up to 0.1% nitrogen in small aggregates or platelets that strongly absorb ultraviolet light below 320 nm. The thermal conductivity of this type of diamond is less than 9 W/(cm-K) at 25°C and its electrical resistivity is greater than 1014 W-m. Almost all industrial synthetic diamond is Type 1b, which contains up to 0.2% nitrogen dispersed throughout the crystal. Low concentrations of nitrogen give diamond a yellow color, whereas higher concentrations lead to shades of green. Colorless Type 2a diamond is almost free of nitrogen and has the best optical and thermal properties of all diamond types. It transmits light at all wavelengths longer than 225 nm (except for some absorption in the 3-5 µm region) and has a thermal conductivity near 20 W/(cm-K) at 25°C. Type 2b diamond is also free of nitrogen, but contains small quantities of boron that give it a blue color and p-type conductivity (resistivity 0.1-10 W-m).
Diamond possesses the greatest thermal conductivity of any material at temperatures near 300 K, which makes it an ideal candidate for applications involving thermal shock (missile domes). The conductivity of diamond is 2-3 orders of magnitude greater than that of other infrared-transmitting materials (Table 1). The measured conductivity of high quality, microwave plasma CVD diamond films [2] peaks near 150 K at a conductivity of 30 W/(cm-K), with room temperature conductivity near 17 W/(cm-K). Conductivities as high as 10-13 W/(cm-K) near 300 K have been reported for other chemical-vapor-deposited diamond samples. [3] Type 2a natural diamond has a thermal conductivity near 13 W/(cm-K) at 500 K and 0.4 W/(cm-K) at 120 K. [4]
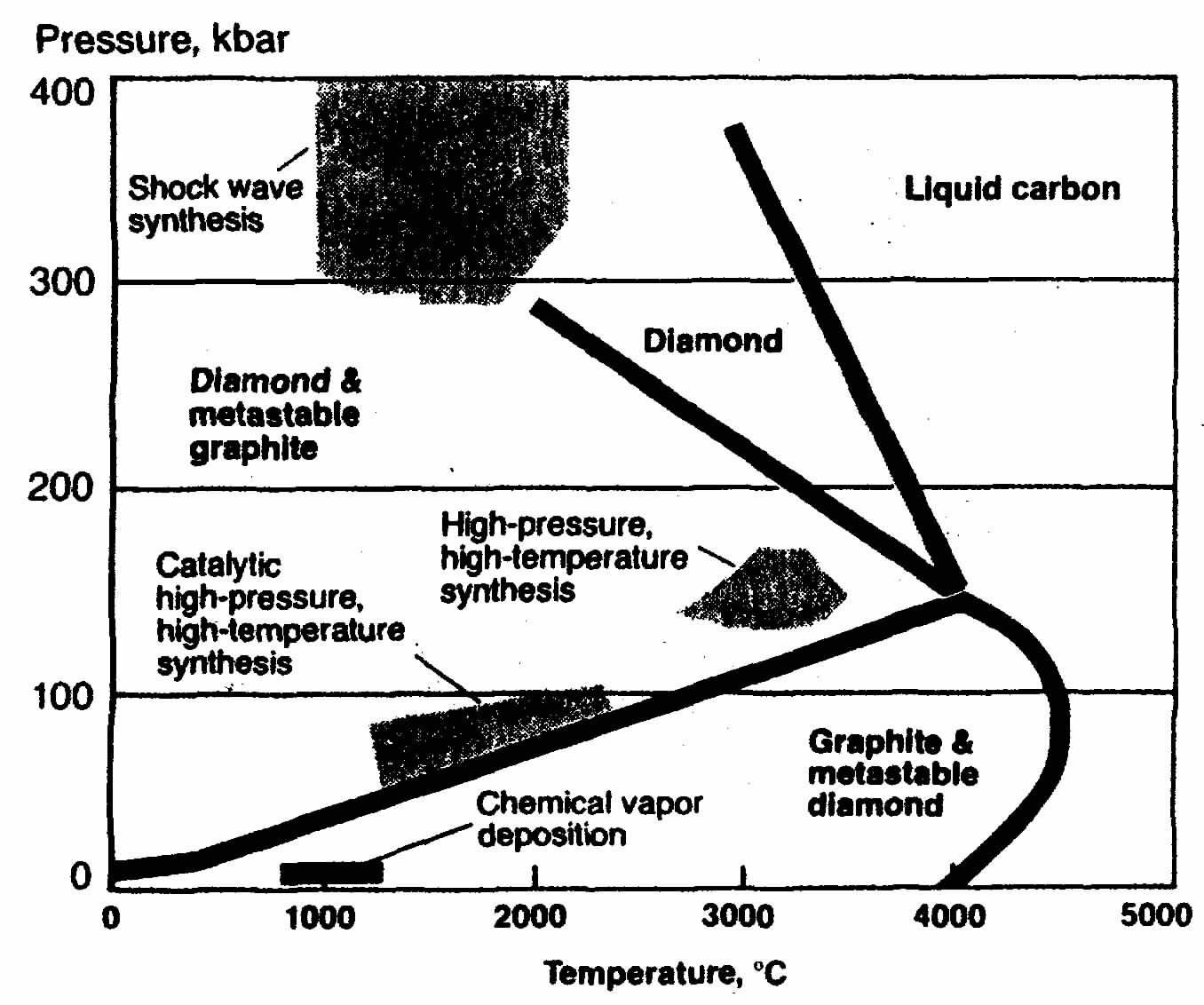
Figure 1. Pressure-Temperature Phase diagram of carbon.
The properties of polycrystalline diamond in diamond films can be quite different from single crystal diamond. One example is the effect of grain size on thermal conductivity of diamond. Since heat is carried through a solid by atomic vibrations (phonons), scattering of the phonons at the boundaries of small grains decreases the thermal conductivity. While grain size is a major factor at low temperatures (at 100 K an increase in size from 0.001 mm to 1 mm crystals implies a 104 increase in thermal conductivity [5]), there is very little dependence on grain size at elevated temperature (500 K) where thermal conductivity is limited by phonon-phonon interactions instead of grain boundary scattering. The grain size of diamond grown by chemical vapor deposition is small at the substrate and increases with increasing distance above the substrate, as does the thermal conductivity.
Diamond Synthesis. All of the known forms of carbon, including graphite, diamond, and fullerenes are formed in nature. Graphite and fullerenes are stable at room temperature, whereas diamond is metastable (Fig. 1). Graphite, in its many forms, is most easily formed through pyrolysis of hydrocarbon materials. Fullerenes are formed under very special conditions of temperature and low pressure in the gas phase. Diamond is naturally formed at conditions where it is the stable form of carbon at high temperatures and very high pressures. Although diamond is thermodynamically unstable at any temperature close to room temperature, heating to over 1500°C is required before the rate of conversion of diamond to graphite is significant. [6] At 2100°C, the rate of this graphitization process converts a 0.1 carat (l carat = 0.2 g) octahedral crystal of diamond to graphite in less than 3 minutes.
A high-temperature, high-pressure process has been employed for industrial synthesis of diamond for many years now. [1] This is a catalytic process where graphite is mixed with molten metals such as nickel, cobalt or iron to produce diamond. Carbon atoms dissolved in the metal under pressures of 60-100 kbar and 1500-2500°C have sufficient mobility to recrystallize from the graphite structure into the diamond structure, which is the thermodynamically stable form of carbon at these conditions. This type of diamond synthesis usually produces micrometer-size powders, but carefully controlled conditions can yield single crystal, high quality diamonds up to 5 carats in mass.
Polycrystalline diamond is another form of synthetic diamond that is widely used in cutting tools. [1] It is manufactured by sintering diamond powder immersed in cobalt at pressures of 50-100 kbar and temperatures of 1200-1600 K. Diamond crystallites grow together catalytically to form a solid that has a grain size of 2-25 µm with residual cobalt inclusions. Cutting tools are typically made with a thick (0.7 mm) layer of this type of polycrystalline diamond on a base of tungsten carbide.
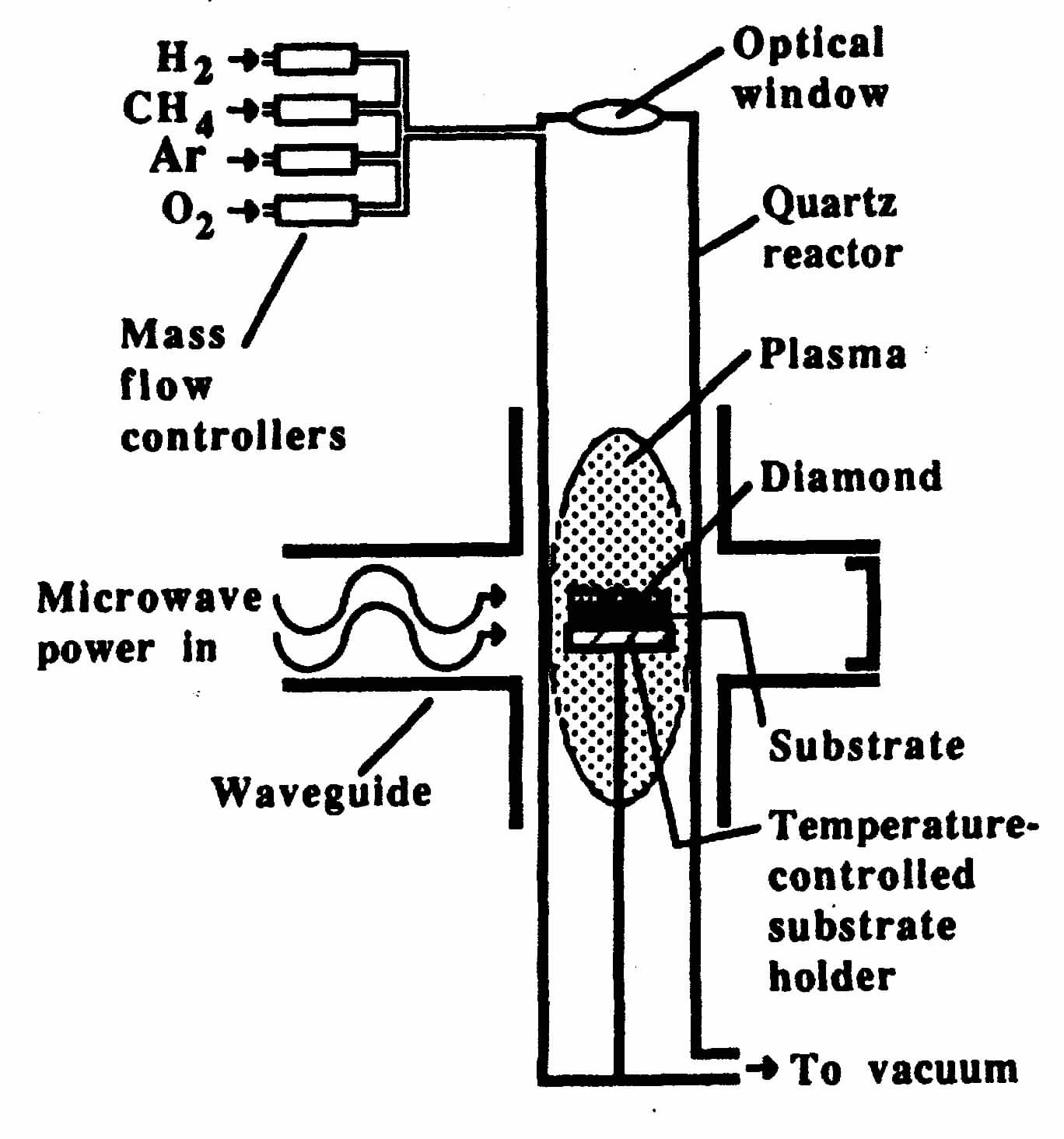
Figure 2. Schematic of a microwave plasma reactor for CVD diamond film growth.
Chemical Vapor Deposition (CVD) of Diamond A major addition to the techniques for diamond synthesis occurred when continuous polycrystalline diamond films were grown over several square centimeters by low pressure chemical vapor deposition. [7] This process does not require the extremely high pressures of other methods; processing pressures are less than 1 atm. Processing temperatures are also somewhat less. Typical conditions might be 950°C and 20 torr, which can be obtained with standard equipment. Although the chemical vapor deposition of diamond occurs at temperatures and pressures where graphite is the stable form of carbon, the chemical environment of the reactor stabilizes diamond relative to graphite.
Figure 2 shows a microwave plasma reactor in which diamond is grown on a silicon substrate. A gas mixture that is typically 99 volume percent hydrogen and 1 percent methane, often with additives such as oxygen and argon, is passed through a quartz tube inside a microwave waveguide. The microwave radiation partially dissociates the gas into a plasma containing hydrogen atoms, methyl radicals (CH3), high energy electrons [8] and other reactive species such as hydroxyl radicals (OH).
Diamond crystallites nucleate on the substrate and grow into a continuous polycrystalline mass. The outer diamond growth surface is rough, but the film face next to the substrate is as smooth as the original silicon surface. High quality Type 2a diamond with a thickness greater than 1 mm can be grown over areas greater than 100 cm2 with growth rates of approximately 0.1 mm/h by this technique.
The principal requirements for diamond deposition in this system are dissociation of the mixture of hydrogen and methane into atoms and radicals at elevated temperature, and crystal growth at lower temperature. A variety of techniques can be used to excite the gas phase molecules. Arc discharges between two high voltage electrodes [9] and dc torches produce high quality diamond at rates of several hundred µ m/h over small areas, or at lower rates over large areas. [10] Using a dc torch, the substrate is cooled to approximately 900°C so that the temperature is not too high for diamond growth.
Two other common means of creating the proper gas conditions for diamond deposition are a hot filament and an acetylene torch. In a hot filament reactor, the gas is heated to approximately 2200°C by a tungsten filament held 5-10 mm from the substrate, whose temperature may be 700 - 1000°C. At a pressure of 20 torr, 1-10% of the hydrogen molecules in the 1% methane /99% hydrogen mixture are dissociated into hydrogen atoms. [11] An advantage of this type of reactor is that it can be directly scaled up to large sizes and irregular shapes. A disadvantage is a relatively slow film growth rate: 0.1-1 µ m/h. An acetylene welder's torch with a 1:1 mole ratio of acetylene and oxygen has also been used to grow diamond films and filaments.
Diamond may be grown on a number of carbon forming substrates such as silicon, molybdenum, and tungsten. Although methane is most commonly used as a precursor material, almost any organic compound [12] may be the source of carbon for diamond growth. Methane is not a necessary ingredient, although methyl radicals formed in the gas phase may be the immediate precursor to diamond growth. [13],[14]
Diamond Heat Shields/Sinks. Single crystal diamond has a higher strength and a larger thermal conductivity than any other known material. Its closest competitor is copper, which has a thermal conductivity of 400 W/m-K at room temperature, a factor of 5 below diamond. Diamond thin heat spreaders are already replacing other materials as heat sinks for electronics. The general relevant properties of diamond are given in Table 1.
Table 1. Properties of diamond [5]
Hardness (kg/mm2) ------------ 9000 Thermal expansion (x 106, K-1) -----------------------0.8
Strength (MPa) --------------- 2500-3000 Thermal conductivity [W/(m-K)] -------------------2000
Young's modulus (GPa) --------1050 Thermal shock figure of merit [R'x 103, W/m] ----5400
A lesser known capability is its ability to sustain thermal shock. A simple thermal shock figure of merit can be used for a qualitative comparison of the thermal shock resistance of different materials [5]. The larger the figure of merit, the greater the heat flux that can be withstood by the material without catastrophic failure. The severe thermal shock figure of merit, R, is
R = S(1-n)/aE
The mild thermal shock figure of merit is
R' = S(1-n)k/aE
where S is the strength of the material, n in Poisson's ratio, a is the expansion coefficient, E is Young's modulus, and k is the thermal conductivity. Table 1 indicates why diamond is the material of choice in applications that are limited by heat transfer capabilities and thermal stress performance - its thermal shock figure of merit is orders of magnitude higher than optical materials and metals as a result of its high Young's modulus.
DEVELOPMENT PLAN
The work plan for this project provides the detailed experimental and modeling foundation for the development of a sintering-type process for joining diamond. The physical and optical characteristics of a system that can be used to grow diamond films by photofragmented C60 CVD growth will be defined. Experiments will be performed that will experimentally demonstrate the growth of a diamond film using this CVD method to clearly define and explore the diamond growth process under conditions that permit relatively straightforward analysis of the grown material. An experiment in bulk diamond growth will be designed based on the film growth results and research into the growth process. Experiments will then be performed that will demonstrate the feasibility of bulk diamond sintering. The experimental results will be analyzed and a feasibility assessment of the overall process will be performed. Modeling of the C60 fragmentation and diamond growth process will be pursued concurrently with the experimental work. The primary questions to be answered by the proposed research are:
1) Can the proposed CVD growth process be used to create high quality diamond films?
2) What are the proper range of processing parameters?
3) How can the growth method be used in a sintering-like process to create volumetric diamond?
4) To what degree is non-diamond carbon included in the material?
5) Does the final bulk porous diamond material have the thermal conductivity and other characteristics of true diamond?
Diamond Sintering Theory. The ongoing models and experimentally demonstrates diamond deposition to bond separate pieces of diamond. The deposition and bonding is achieved by the CVD of linear, multi-atom carbon molecules generated by photofragmentation of fullerene vapor driven by pulsed laser illumination. Standard CVD diamond growth cannot be performed in the small volumes required for bonding/sintering, because the plasmas normally used to create carbon-containing radicals cannot be created in such small volumes. Using an intense laser source, however, fullerenes can be fragmented for diamond growth before the excited molecules are collisionally quenched by the solid walls. The laser radiation can penetrate the fullerene vapor-saturated volume as a result of the transparency of the surrounding diamond and a transparent container. The basis for the credibility of this approach rests on separate work that shows that 1) Diamond growth can be performed using fullerene fragmentation in a Argon microwave plasma, even without the addition of hydrogen [15],[16] 2) C60 photofragmentation occurs in response to excitation by a high intensity light source, and the fragmentation occurs in preference to ionization as a result of the unusual nature of the resonant bonding in the fullerene molecule [17],[18]. Since both the photofragmentation and diamond deposition of C60 are critical to the feasibility of this effort, they are discussed in detail.
Fullerenes are a form of pure carbon that have carbon atoms in an alternating hexagonal/pentagonal sites that form a closed ball structure that compliments the planar form of carbon (graphite) and the tetrahedral carbon crystal known as diamond. Fullerenes are unique as a carbon form as a result of their high vapor pressure and their high solubility in common solvents. Fullerenes are potential precursors in many plasma chemistry and chemical vapor deposition processes. They are also potential sources of high intensity cluster beams for ion implantation and surface modification. The fragmentation and ionization processes that the fullerenes undergo will, to a large degree, determine the outcome of such reactions.
Fullerene Diamond Film Growth - This discussion is based on the work of Gruen et. al. [15],[16] that demonstrated that diamond thin films could be grown using C60 fullerene as the precursor in the standard plasma-enhanced chemical vapor deposition (PECVD) process. The extensive fragmentation of C60 to C2 in an argon plasma observed by Gruen et. al. led them to this investigation of the use of C60 as a precursor for CVD growth of diamond films.
They began by attempting diamond film growth using C60 as a precursor in a H2 atmosphere [15]. Fullerene-containing soot was placed in an oven, heated to 550°C, and an Ar/H2 mixture (20 sccm Ar, 4 sccm H2) was passed through the sublimator into the plasma chamber while maintaining a total pressure of 100 torr. A 76 mm diameter single-crystal silicon disk, which had been mechanically treated with 0.1 µ m diamond powder, was placed on a graphite holder and maintained at 850µC during the experiment. A 1500 W microwave discharge was maintained in the gas mixture during a 16 h deposition. Optical emission measurement showed intense C2 Swan band emission, as well as intense Ha and Hb but relatively much weaker Ar emission lines. The Si substrate was examined after the deposition using scanning electron microscopy (SEM), x-ray diffraction (XRD), and Raman spectroscopic techniques to verify diamond deposition [16].
Although it is known that hydrogen incorporated in the diamond lattice during growth introduces defects, hydrogen was believed to be an absolutely necessary constituent for CVD diamond growth, based on experimental results [19] and the theoretical elaboration of growth mechanisms. [19],[20],[21] Although diamond films have been grown from gas mixtures without molecular hydrogen, significant concentrations of hydrogen were furnished either by the hydrocarbon constituents themselves or by water vapor (e.g. [22]). CH4-Ar and CH4-He systems [23] have also been employed, but resulted only in graphite or diamondlike carbon films. Hibshman [24] explored the lower limit of hydrogen required for diamond formation in the CO-H2 system and found it to be 0.5%.
In the same apparatus, fullerene-containing soot which had been treated with methanol to remove hydrocarbon constituents and which had subsequently been thoroughly degassed, was placed in a sublimator located in a sidearm of the plasma deposition chamber. [16] IR examination of C60 films sublimed onto Si wafers showed only C60 absorptions, indicating that the soot contained negligible amounts of hydrogen. Fullerene vapor was carried into the plasma chamber by flowing Ar gas through a sublimator that was kept at 550°C. Assuming a saturated vapor of pure C60, the estimated atomic carbon content of the Ar carrier gas was 0.14%. The Ar flow rate was regulated at 20 sccm, while the total pressure in the chamber was controlled at 100 Torr. Single-crystal silicon (100) substrates mechanically polished with 0.1 µ m diamond powder were placed on a graphite stage covered with a silicon wafer. Under these conditions, no film growth was observed in a fullerene-free argon plasma. Deposition was carried out at 850°C with 1500 W of microwave power for 1-3 h. The as-deposited films are uniform, reflective, and highly resistive. Based on cross-section scanning electron microscope (SEM) images, it is estimated that the diamond growth rate was about 1.2 ° m/h, which is higher than or at least comparable to that commonly observed using 1% methane in hydrogen as a precursor under similar deposition conditions.
It was concluded that a growth mechanism not dependent on hydrogen abstraction reactions must be at work. [16] In situ optical measurements reveal very intense C2 Swan band (d 3Pg - a 3Pu) emission, which is believed to be a result of collisional and other fragmentation processes of the C60. [25] The C2 fragments are thought to be the primary precursor species for fullerene diamond growth. The Swan band emission is the first experimental indication that C60 fragmentation occurs as a result of successive elimination of (undetected) neutral C2 groups. [26]. Many studies have shown that C60 photofragmentation results in even-numbered clusters in the range C60 - C32 (e.g. [27]). With this approach, rate constants for fragmentation and for delayed electron emission have been deduced. [18]
Characterization The diamond films that were grown were characterized in a number of ways. The existence of diamond-like-carbon (DLC) films that have very similar properties to diamond films [5] necessitate the careful analysis of the grown films to verify the formation of diamond without other types of carbon impurities. The analytical techniques and their results for diamond and DLC are thus important for the proposed program. Diamond films are analyzed using of x-ray diffraction (XRD), electron diffraction, transmission electron microscopy (TEM), scanning electron microscopy (SEM), electron energy loss spectroscopy (EELS), Raman spectroscopy, and other techniques. Of these, Raman spectroscopy is the technique that is most often used to demonstrate the existence of diamond because it shows a single peak at approximately 1332 cm-1 as a result of the high symmetry of the diamond lattice. Other forms of carbon show additional bands and a shifted major peak.
To demonstrate the purity and quality of their fullerene-generated diamond films, Gruen et. al. performed a series of measurements on a typical film after 3 h of deposition that illustrate what will be done in this program. Raman spectra showed a band centered at 1333 cm-1 and a broad feature at about 1550 cm-1. The 1333 cm-1 band with a FWHM of about 17 cm-1 is characteristic of bandwidths observed with small-grain size diamond films. [28] The 1550 cm-1 peak is also commonly observed with small-grain size diamond films and is thought to be associated with disordered carbon at grain boundaries. [29] X-ray diffraction showed three major diamond peaks corresponding to the (111), (220), and (311) reflections, respectively, and a TEM electron diffraction pattern from a small area could be fully indexed to the face centered diamond lattice with an average lattice constant of 3.53 A. This agrees within error with that obtained from single-crystal diamond (3.56 A via XRD). TEM imaging showed a fine-grained deposit with a smaller defect density compared to films prepared at 300 mTorr with a magnetized plasma using CH4-H2-O2 mixtures. [30] Intergranular boundaries were shown to be free of graphitic phase. EELS was also used to give supporting results. [31]
Diamond Growth Reaction Chemistry CVD diamond growth has been experimentally demonstrated, but the precise mechanism of growth is still under investigation. It is believed that the dangling bonds of the diamond lattice are capped by hydrogen atoms in all diamond, and that the growth of diamond occurs by insertion of carbon atoms beneath the hydrogens, followed by the bonding of each carbon to its neighboring carbon atoms. Most of the various processes that lead to diamond film growth that have been described previously postulate hydrocarbon gases as precursors. A frequently discussed growth mechanism involves the CH3 radical as the principal growth species [32] particularly when methane is used as the precursor gas. C2H2 has also been invoked as the growth species [33] when acetylene is used as the precursor. Both mechanisms depend on the reaction of atomic hydrogen created in the gas both with the surface hydrogens and with the hydrocarbon species absorbed on the growing surface. Hydrogen abstraction reactions are required for carbon to be incorporated into the diamond lattice when hydrogen containing growth species are used for diamond growth. These hydrogen reactions make the growth process very energy inefficient, since many H2 molecules (10,000 to 1) must be dissociated to allow a carbon insertion.
The mechanism of diamond growth in a fragmenting fullerene gas invokes the insertion of C2 fragments at the surface. The experimentally determined adsorption energy of C2 on graphite surface is very high, 815 kJ, [34] compared to model calculations of approximately 50 kJ for C2H2 adsorbing on a C22H27 template simulating diamond. [35] The implication is that C2 can relatively easily insert directly into the C-H bonds of a hydrogen-terminated diamond surface. To explore the energetics of such a mechanism for diamond growth, the reaction:
C2(1Sg+) + CH4 --> H3CHCC --> H3CCCH
can form an oversimplified model for addition of C2 to a diamond surface. The first step corresponds to insertion of singlet C2 into the C-H bond. The second step corresponds to migration of the hydrogen to the terminal carbon.
Preliminary results [15] from low level calculations (ab initio molecular orbital theory, HF/3-21G) of the potential energy surface suggest little or no barrier for the first step of reaction (1), i.e., insertion of C2 into a CH bond of methane to form H3CHCC. The barrier for isomerization of H3CHCC to H3CCCH, the second step in reaction (1), is about 3 kcal/mol at the HF/3-21G level. Generally, these types of barriers are reduced at higher levels of theory. With C2 as the growth species, the 13-step hydrocarbon precursor growth mechanism proposed by Belton and Harris [33] could be reformulated as a 5-step mechanism with the first two steps being successive C2 insertion reactions. The remaining three steps would then be identical to steps 11-13 of Belton and Harris. [33] The 5-step C2 mechanism strongly reduces the requirement for hydrogen abstraction reactions and this, coupled with rapid C2 insertions, could lead to improved growth kinetics. This modeling confirms the experimental data that show that fullerenes can serve as precursors for CVD diamond film growth without the addition of hydrogen or oxygen.
Fullerene Photofragmentation. Wurz and Lykke [17],[18] have investigated the processes of fullerene photofragmentation and photoionization. The interaction of intense laser light in the visible and UV wavelength range with gas-phase C60 leads to high internal excitation (~50 eV) of the C60 molecule rather than direct multiphoton ionization. C60 has a very rigid and highly symmetric molecular structure that has a very high density of vibrational states and thus undergoes an extremely rapid thermalization (10-14 s) of a photoexcited electronic state. For this reason the neutral C60 molecule has been shown experimentally and theoretically to absorb 10-20 photons to form a superexcited molecule, even though 2-3 photons are sufficient to achieve multiphoton ionization (7.6 eV ionization energy). Delayed ionization (time scale 1-10 ms) and fragmentation both have high internal excitations as common precursors and both were found to occur at about the same rates.
In their experimental work a wide fluence and wavelength range was investigated to map out the different parameters that characterize these processes. A Ta cup filled with approximately 200 mg of pure C60 was held at a temperature of 800 K to give a vapor pressure of about 5 x 10-4 Torr. Laser fluences from 0.01 to 103 mJ/cm2 were used at wavelengths of 212, 266, 355, and 532 nm. Vacuum UV excitation was found to cause prompt, direct photoionization, whereas lower energy photons caused a combination of fragmentation and delayed (thermionic) ionization. They were unable to detect the low-mass neutral products of the fragmentation. As long as the photon energy was less than that needed for direct ionization, high fluence laser irradiation of C60 resulted in thermal fragmentation and delayed ionization with equal efficiencies. Thus for submicrosecond laser pulses photofragmentation is the dominant result of laser energy absorption by C60. Furthermore, C60, is a non-linear optical material whose absorption increases by a factor of 3-5 for its excited states.
Further work [36] has shown that other carbon fragments (C, C3, C4) are also generated during the breakup of the C60 molecule. Theoretically, 10-12 eV should be required for C2 loss from C60 dissociation, whereas experimentally, only 5-7 eV are required. Apparently the fragmentation process is complex, affected by the fullerene structure at the time of dissociation, which can have many forms, as its effective temperature increases. None of this alters the experimental demonstration of the growth of diamond from fragmented fullerenes.
Based on previous C60 CVD diamond film growth experiments, the preferred temperature of the substrate and vapor source for experiments is 800-850°C. This is the established optimum for CVD diamond growth, and it coincidentally maximizes the vapor pressure of C60 (Fig. 3) while remaining below its decomposition temperature
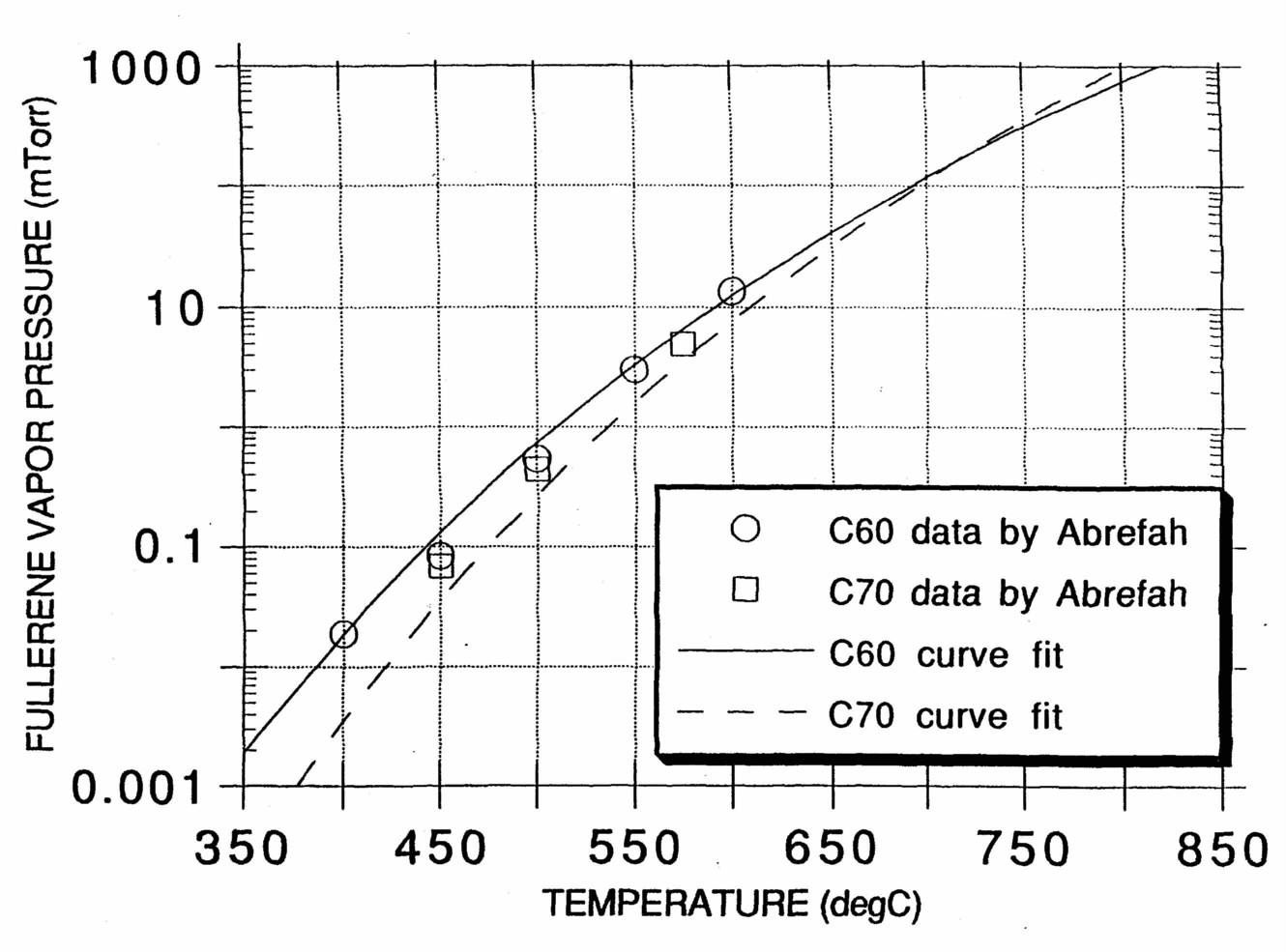
Figure 3. Fullerene vapor pressure as a function of temperature [27].
(about 900°C [26]). By maintaining a uniform temperature throughout the CVD chamber C60 vapor can be prevented from condensing and a relatively high concentration can be maintained in the gas phase (about 1 torr).
The specification of the optical excitation source is dictated less by the photon fluences required for fragmentation, or the geometry of the film growth experiments than it is by length scale of the volume in which fragmentation and deposition must take place. Conventional diamond deposition takes place in an open chamber above a substrate on which diamond is grown. For bonding/sintering, the length scale is much smaller and forms a severe constraint for excitation by fragmentation before collisional deexcitation. To achieve diamond bonding, the length scales must be small to be compatible with the micron/hr growth rates typical of CVD diamond growth.
Assuming a 10 µ m diameter separation, and a 800°C temperature, the velocity of a C60 molecule is on the order of 104 cm/s, and the average time before wall collision is on the order of 10 ns, which is a convenient pulse length for a high power Q-switched laser. The minimal laser flux can then be estimated by a conservative 50 eV of energy must be pumped into a C60 molecule with an 11 A diameter to fragment it. Thus 10-17 joules in a cross sectional area of 10-18 m2 is required; on the order of 10 J/m2. An Alexandrite laser (Light Age, Inc. Model 101-PAL) provides a second harmonic generated (SHG) 0.1 J pulse energy, 20 ns pulse length, and 20 Hz repetition rate in the wavelength range of 360 - 400 nm. A spot size of 1 cm2 would give the needed 10 J/m2, and a good size for deposition experiments. The deposition rate is controlled by the C60 vapor density and the fragmentation rate caused by the laser pulses.
Deposition geometries, appropriate optical excitation sources, and diamond growth parameters. It seems probable that forming diamond across a small gap between two already existing diamond pieces would be the best way to perform the bonding. For maximum strength and material continuity, the deposited diamond must form on both surfaces and grow together. The deposition growth must be supplied from the gas, so a build up of a wide angle would be best, rather than needing to transport carbon fragments down a narrow crevice. Diamond growth rates will be controlled by the feed of fullerene vapor, but may also be controlled by the fragmentation dynamics. Research has shown that morphological control of growth is critical for the formation of high quality diamond films [37].
Open deposition on a flat substrate that will be performed to demonstrate diamond growth can be performed by a lower power light source; perhaps even by a UV lamp. The requirements for such a source will be investigated, with the intent of minimizing the cost of this demonstration source.
The basic experimental components are a light source and light transfer optics, a vacuum vessel with optical access, a fullerene source, ancillary gas sources, and a growth substrate. An apparatus will first be designed to demonstrate fundamental diamond growth capabilities.
DIAMOND FILM GROWTH
The "sintering" concept will first be developed by growing diamond on diamond grit dispersed on a silicon wafer. Key parameters will include grit size, substrate temperature, particle-particle distance, and height of the grit layer. SEM will determine how the growth proceeds and how far the "sintering" extends into the stacked grit layers. We will also take a systematic approach using the mosaic growth technique of Geis et al[38]. Using their approach, we will pattern and etch a regular array of pyramidal pits into a Si wafer and then disperse diamond particles into the pits. This produces an array of diamond particles held at specific distances from each other. Growth on these arrays will give us valuable information on specific packing densities required for successfully "sintering" monolithic samples.
One experimental configuration is shown in Fig. 4. The entire apparatus would be insulated and kept isothermal at 800°C using standard heating coils and a variac or SCR temperature controller. Thermal equilibrium will be maintained by radiant heating, which is quite significant at this temperature and will maintain heat transfer in the vacuum. The substrate can also easily be heated with a resistance element, and this capability will be built in. The vacuum vessel will be quartz (working temperature over 1000°C), surrounded by an insulated heating chamber that has axial ports for vacuum/low pressure regulation, and laser access. The fixture would be small and portable to allow apparatus development followed by transport to the laser facility for optical excitation. A C60/C70 mixture (typically 85% C60) will be used because it is much cheaper, and primarily C60 will be produced as a result of its higher vapor pressure.
The chamber will be evacuated and then heated, and the vessel will be filled with C60 vapor. C60 condensation on cold surfaces can be prevented by back filling with argon to 1 Torr. The entire chamber could be filled with argon, but gas diffusion times can be very long. Rapid experiments can be done under vacuum. Care will have to be taken to keep oxygen out of the apparatus because C60 reacts rapidly at high temperatures. Furthermore, no C60 solid films can be allowed to build up on the laser illuminated surfaces, because the illumination will polymerize the film into an inert graphitic structure [39].
The type of substrate will be either Si or diamond. Si substrates abraded with diamond powder are commonly used and have the advantage of not interfering with the analysis of the grown films. The alternative is a diamond macle that would ensure rapid nucleation but would make Raman analysis problematic. Another motivation for using a diamond substrate would be to determine if there is preferential growth on a (111) face compared to (100) face.
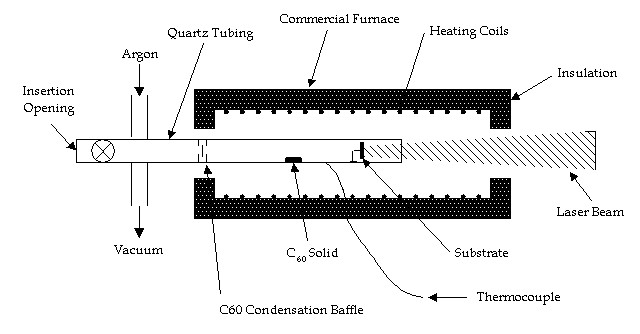
Figure 4. Apparatus schematic for diamond film growth using C60 photofragmentation chemical vapor deposition.
The grown films will be sent to CWRU for detailed analysis with the appropriate techniques: Raman spectroscopy, SEM, XRD, and Atomic Force Microscopy (AFM).
The work of this task will be to carry out this design in much greater detail. The furnace, the quartz vacuum cell, the substrate and holder, and the vapor source will be constructed as a portable unit that can be taken to the laser facility for intermittent experiments. Initial debugging of the apparatus can be done away from the Laser Facility. The cell can be cleaned and used multiple times for repeat experiments. Two cells will be constructed for multiple experiments at the laser facility.
Diamond vs. Non-diamond Carbon . The issue of whether the grown films are true diamond or some sort of pseudodiamond is complex. Dr. Gruen's group at Argonne has been growing diamond films from fullerenes for almost 10 years and has characterized the diamond films exhaustively, in the process clarifying the tests necessary to demonstrate that true diamond has been grown. All testing indicates that fullerene grown diamond films are indeed true diamond. The grain size in the diamond films is smaller (nm) than other types of CVD diamond, but this type of film gives superior properties to the films for some applications.
BULK DIAMOND SINTERING
The basic experimental arrangement for fabrication of bulk porous diamond will be as shown in Fig. 5, where a quartz cell will contain the C60/C70 mixture at the bottom, a layer of quartz beads, and on top of that a layer of diamond grit. The entire container will be fairly small to minimize the mass of fullerenes and diamond grit necessary. The quartz cell will be heated to a temperature where the appropriate vapor pressure of C60 is obtained. The outer box will be evacuated to high vacuum and backfilled with Argon a few times to be sure oxygen levels are negligible during the experiments. The quartz container containing the powder, beads, and diamond grit must be kept at constant temperature. The figure shows a heated sapphire window on top of the quartz container to contain the fullerene vapor and prevent condensation on a cold surface. Build-up of deposits on this window will have to be avoided. It may be more practical to heat the entire inside of the larger box and rely on the lower laser intensity on the upper window to prevent build-up.
There are a number of possible sources of diamond grit. The requirements are a minimum of 10 mm diameter, and a transparency similar to Type 1 diamond (transparent for wavelengths longer than 320 nm). Standard industrial diamond grit may be adequate. Although not the best diamonds, this material is cheap ($1/carat) and up to 10 µ m in diameter; this size may be marginal but adequate to create 10 µ m pores. Diamond from CVD diamond experiments may be adequate also.
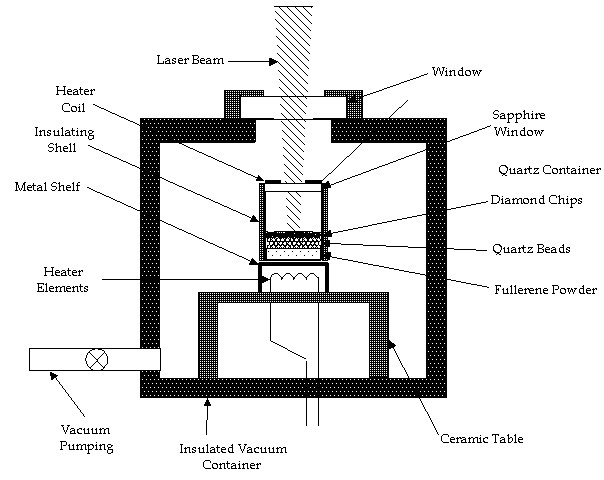
Figure 5. Schematic of bulk diamond fabrication apparatus.
The major issue to be addressed in the process design for making bulk diamond is how to uniformly deposit diamond throughout the bulk. Scattering of light by the particles will distort penetration of the laser beam. Too rapid a deposition at the upstream end may block further C60 flow through the bulk as well as depleting the C60 concentration for downstream deposition. Concentration of the light at the downstream end, moving toward the upstream end is one solution. Large parts could also be made layer by layer, where the thickness of the layer would be determined by the depth at which the particle scattering diffuses the laser pulse too much to achieve the necessary intensity for fragmentation. The processing may also take place in a batch mode, such that C60 vapor first fills the part, it is fragmented and deposited by one or a number of laser pulses, and then the vapor concentration is then raised to the desired level again throughout the part.
Particle size optimization may be important also, in a similar fashion to sintering, where particles are typically in the size range of 10-100 µ m, and football shaped particles seem to be the optimum. If attachment occurs for the first collision the crevices will tend not to be filled and a columnar initial powder structure would be best.
The initial goal of experiments will be to demonstrate some cohesion of the diamond grit after the chip mass is removed and residues dissolved. Once cohesion is demonstrated efforts will be made to verify that the separate diamond pieces are joined by diamond material and that the bulk has significant strength. The final goal is to obtain major connections between the grit particles such that the entire mass exhibits diamond properties.
The production of bulk diamond will be indicated by a combination of strength and the demonstration that only diamond is present and not other forms of carbon. Once the porous diamond has been bonded, the piece will be treated to remove residue and analyzed. One complication is that other carbon materials might be fully enclosed in voids, and confuse the analysis. This can be avoided by analyzing the process in its early stages. Mass analysis using a microbalance may also be used to determine growth rates.
Once a piece of diamond is grown, it will be tested for its thermal conductivity. The effect of porosity should be calculable based on the many porous materials commercially produced. Although detailed measurements will not be made it should be obvious based on the capacity of the material for heat flow whether true bulk diamond has produced.
REFERENCES
1. J. Wilks and E. Wilks, Properties and Applications of Diamond, Butterworth Heineman, Oxford, (1991).
2. D. T. Morelli, T. M. Hartnett, and C. J. Robinson, "Phonon-Defect Scattering in High Thermal Conductivity Diamond Films," Appl. Phys. Lett., 59, 2112 (1991).
3. E. P. Visser, E. H. Versteegen, and W. J. P. van Enckevort, "Measurement of Thermal Diffusion in Thin Films Using a Modulated Laser Technique: Application to Chemical-Vapor-Deposited Diamond Films," J. Appl. Phys., 71, 3238 (1992).
4. D. G. Blanchard, E. E. Marotta, and L. S. Fletcher, "A Survey of the Thermal Conductivity of Synthetic and Natural Diamond Materials," Am. Inst. Aero. Astro. Paper AIAA 92-0708, 30th Aerospace Sci. Meeting, Reno, Nevada, (1992).
5. D. C. Harris, "Infrared Window and Dome Materials," SPIE Press, TT 10, (1992).
6. T. Evans and P. F. James, "A Study of the Transformation of Diamond to Graphite," Proc. Roy. Soc. London, A277, 260 (1964).
7. J. C. Angus, Y. Wang, and M. Sunkara, "Annu. Rev. Mater Sci., 21, 221 (1991).
8. F. M. Cerio and W. A. Weimer, "Electrostatic Probe Measurements for Microwave Plasma-Assisted Chemical Vapor Deposition of Diamond," Appl. Phys. Lett., 1991, 3387 (1959).
9. F. M. Cerio and W. A. Weimer, "Construction of an Inexpensive dc Plasma Jet Chemical Vapor Deposition using Commercially Available Components," Rev. Sci. Instrum., 63, 2065 (1992).
10. N. Ohtake and M. Yoshikawa, "Diamond Film Preparation by Arc Discharge Plasma Jet Chemical Vapor Deposition in the Methane Atmosphere," J. Electrochem. Soc, 137, 717 (1990).
11. K.-H. Chen, M. -C. Chung, C. M. Penney, and W. F. Banholzer, "Temperature and Concentration Distribution of H2 H Atoms in Hot-Filament Chemical-Vapor Deposition of Diamond," J. Appl. Phys., 71, 1485 (1992).
12. W. A. Weimer, F. M. Cerio, and C. E. Johnson, "Examination of the Chemistry Involved in Microwave Plasma Assisted Chemical Vapor Deposition of Diamond," J. Mater. Res., 6, 2134 (1991).
13. D. G. Goodwin and G. G. Gavillet, "Numerical Modeling of the Filament-Assisted Diamond Growth Environment," J. Appl. Phys., 68, 6393 (1990).
14. M. P. D'Evelyn, C. J. Chu, R. H. Hange, and J. L. Margrave, "Mechanism of Diamond Growth by Chemical Vapor Deposition: Carbon-13 Studies," J. Appl. Phys., 71, 1528 (1992).
15. D. M. Gruen, S. Liu, A. R. Krauss, J. Luo, and X. Pan, "Fullerenes as precursors for diamond film growth without hydrogen or oxygen additions," Appl. Phys. Lett., 64, 12, 1502 (1994).
16. D. M. Gruen, S. Liu, A. R. Krauss, and X. Pan, "Buckyball microwave plasmas: Fragmentation and diamond-film growth," J. Appl. Phys., 75, 3, 1758 (1994).
17. P. Wurz and K R. Lykke, "Multiphoton Excitation, Dissociation, and Ionization of C60," J. Phys. Chem., 96, 10129 (1992).
18. Y.L. Yang, I.M. Struck, L.E. Sutco, and M.P. D'Evelyn, "Chemistry of hydrogen on diamond (100)," Thin Solid Films, 225, 203 (1993).
19. Diamond Films and Coatings, P. E. Pehrsson, F. G. Celii, and J. E. Butler, (Noyes, Park Ridge, NY, 1993), vol. 68.
20. S.J. Harris and D.C. Goadwin, "Growth on the Reconstructed Diamond (100) Surface," J. Phys. Chem., 97, 23 (1993).
21. J.A. Mucha, D.L. Flamm, and D.E. Ibbotson, "On the role of oxygen and hydrogen in diamond-forming discharges," J. Appl. Phys., 65, 3448 (1989).
22. O. Matsumoto and T. Katagiri, "Effect of Dilution Gases in Methane on the Deposition of Diamond-Like Carbon in a Microwanve Discharge II: Effect of Hydrogen," Thin Solid Films, 146, 283 (1987).
23. H. J. Hibshman, "Diamond Growth Process," USA Patent #3371996, (1968).
24. D.J. Gruen, "Conversion of fullerenes to diamond.," Nucl. Instrum. Methods in Phys. Res. B, 78, 118 (1993).
25. S.C. O'Brien, J.R. Heath, R.F. Curl, and R.E. Smalley, "Photophysics of buckminsterfullerene and other carbon clusters," J. Chem. Phys., 88, 220 (1988).
26. P. Wurz, K.R. Lykke, M.J. Pellin, and D.M. Gruen, "Velocity distributions and photodissociation of neutral C60 and C70 clusters," J. Appl. Phys., 70, 6647 (1991).
27. L.H. Robbins, E.N. Farabaugh, and A. Feldman, "Growth of diamond films by hot filament chemical vapor deposition," J. Mater. Res., 5, 2546 (1990).
28. J. Fink, T. Muller-Heinzerling, J. Pfluger, et. al., "Structure and Bonding of Hydrocarbon Plasma Generated Carbon Films: an Electron Energy Loss Study.," Solid State Commun., 47, 687 (1953).
29. D. B. Gruen, X. Pan, A. R. Krauss, S. Liu, J. Luo, C. M. Foster, "J. Vac. Technol., (in press).
30. J.E. Butler and R.L. Woodin, "Thin film diamond growth mechanisms," Philos. Trans. R. Soc. London A, 342, 209 (1993).
31. M. Frenklach and K.E. Spear, "Growth mechanism of vapor-deposited diamond," J. Mater. Res., 3, 133 (1988).
32. S.C. Bates , "Technique for High Mixing Rate, Low Loss Supersonic Combustion with Solid Hydrogen and Liquid Helium Fuel," USA Patent #6,003,300, (21 December 1999).
33. R. J. Thorn and G. H. Winslow, "J. Chem. Phys., 26, 186 (1957).
34. K. Larsson, S. Lunell, and J.-O. Carlsson, "Diamond Relat. Mater., 2, 949 (1993).
35. J. Abrefah and D. R. Olander, "Vapor Pressure of Buckminsterfullerene," Appl. Phys. Lett., 60, 11, 1313 (1992).
36. K.R. Lykke, "Fragmentation of C60: Experimental detection of C1, C2, C3, and C4 by xuv photosionization.," Phys. Rev. A, 52, 2, 1354 (1995).
37. A.L. Yee, H.C. Ong, L.M. Stewart, and R.P.H. Chang, "Development of flat, smooth (100) faceted diamond thin films using microwave plasma chemical vapor deposition.," J. Mater. Res. 12, 7, (1997).
38. M.W. Geis, H.I. Smith, A. Argoitia, J. Angus/G.H.M. Ma, J.T. Glass, J. Butler, C.J. Robinson, and R. Pryor, "Large-area mosaic diamond films approaching single-crystal quality," Apl. Phys. Lett., 58, 22, 2485 (1991).
39. V. Hruby, M. Martinez-Sanchez, S. C. Bates, and D. Lorents, "Fullerene Fueled Electrostatic Thrusters - Feasibility and Initial Experiments," AIAA Paper 94-3240, (1994).